| ENDODONTICS | Editor: Richard E. Walton |
Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment
Göran Sundqvist, DDS, PhD,a David
Figdor, BDSc, MDSc, FRACDS, Dip Endo,b Sten Persson, DDS,
PhD,c and Ulf Sjögren, DDS, PhD,d Umeå, Sweden, and Melbourne, Australia
UMEÅ
UNIVERSITY
Objective. The purposes of this study were to determine what microbial flora were present in teeth after failed root canal therapy and to establish the outcome of conservative re-treatment.
Study design. Fifty-four root-filled teeth with persisting periapical lesions were selected for re-treatment. After removal of the root filling, canals were sampled by means of advanced microbiologic techniques. The teeth were then re-treated and followed for up to 5 years.
Results. The microbial flora was mainly single species of predominantly gram-positive organisms. The isolates most commonly recovered were bacteria of the species Enterococcus faecalis. The overall success rate of re-treatment was 74%.
Conclusions. The microbial flora in canals after failed
endodontic therapy differed markedly from the flora in untreated teeth. Infection at the time of
root filling and size of the periapical lesion were factors that had a negative influence on the
prognosis. Three of four endodontic failures were successfully managed by
re-treatment.
(Oral Surg Oral Med Oral Pathol Oral Radiol Endod
1998;85:86-93)
When teeth are treated by root canal therapy under aseptic conditions and according to accepted clinical principles, the success rate is generally high. Most follow-up studies on endodontic therapy report overall success rates of 85% to 90%.1-8
Although many failure cases are caused by technical problems during treatment, some cases fail even when apparently well treated. A number of factors have been identified as agents associated with failure of endodontic therapy, including extraradicular infection, foreign-body reactions, and true cysts.9-11 However, most treatment failures are caused by microorganisms persisting in the apical parts of root canals of obturated teeth.12 Information on the nature of these infections is sparse, but studies of the microbial flora from the canals of previously root-filled teeth with persisting periapical lesions have revealed that the flora differs markedly from that of untreated necrotic dental pulps.13,14 The microbial flora in the canals of teeth after failed endodontic therapy appears to be a very limited assortment of the microorganisms that have been reported in untreated root canals.15-17 It is unknown whether this apparent process of selection depends on a specific resistance of microorganisms to the antimicrobial measures and medicaments used during treatment or on a particular ability of some microbes to survive in the restricted nutritional environment of the root-filled canal.
In cases in which endodontic treatment fails, it is generally agreed that the optimal approach is to undertake conventional re-treatment, but periapical surgery remains an additional or alternative option for management of cases in which re-treatment is not possible. Whether a root-filled tooth with a periapical lesion requires treatment depends on an assessment of whether the lesion is healing or not. If radiographs from the previous treatment are available for comparison with follow-up radiographs, then the decision about retreatment is made easier. The presence of an incomplete or defective root filling associated with a developing periapical lesion is a clear indication for re-treatment. Similarly, if the root filling appears defective and the tooth requires a new restoration, the prudent choice would usually be to re-treat the previous endodontic treatment. For teeth in which no periapical lesion was present initially, the development of a subsequent lesion warrants that the tooth be re-treated. For teeth with preoperative lesions, the length of the postoperative period is an important factor in deciding whether re-treatment is required; this is because the lesions must be given enough time to heal. A follow-up period of at least 4 years is considered desirable.1
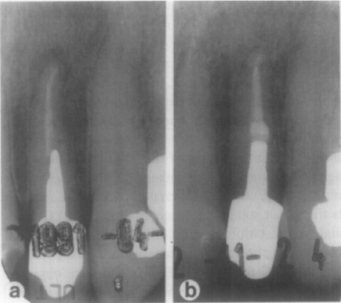
Fig. 1. Successful re-treatment: preoperative and follow-up of lateral incisor. Preoperative (a) shows failing endodontic treatment. Post-crown and root filling were removed, and canal was re-treated and restored. Recall radiograph taken 18 months later (b) reveals periapical healing.
A number of studies1,8,18-20 have retrospectively evaluated the success rates of re-treatment. However, few prospective studies have been established to determine the outcome of endodontic re- treatment. One aim of the present study was to determine the success rate of retreatment and identify factors that might influence the prognosis. Another aim was to obtain data on the composition of the microbial flora in previously root-filled teeth with persisting periapical lesions by means of sophisticated anaerobic culturing techniques. The teeth were re-treated and then followed over 5 years to determine the outcome of re-treatment and to evaluate the factors that might influence the prognosis.
MATERIAL AND METHODS
Clinical material
Fifty-four teeth were selected for re-treatment. All of the teeth were asymptomatic, had been previously root-filled, and showed radiographic evidence of periapical bone lesions. The teeth had histories of root canal treatment more than 4 to 5 years earlier; in some cases the teeth had been regularly followed for 2 years, during which time there had been no signs of healing. Apart from one case that had been poorly obturated, all teeth had root fillings that appeared to reach a reasonable radiographic standard. Figs. 1 and 2 are representative of the radiographic standard of most root fillings in this study. Five of the teeth were two-rooted with two root canals; other teeth had one root canal. Radiographic examination was by the paralleling technique with 22 × 35 mm Kodak Ultraspeed film (Kodak) in a film holder.21 The size of each lesion was calculated as an average of the lesion’s largest dimension and the perpendicular to the largest dimension.
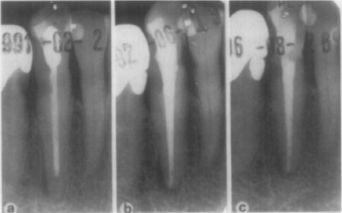
Fig. 2. Failed re-treatment: preoperative and follow-up of canine. Preoperative (a) reveals failing endodontic treatment. Canine has radiolucent restoration at distal cervical margin; adjacent first premolar tooth has carious lesion that was subsequently restored with amalgam. Endodontic treatment was redone, but review radiograph taken 15 months later (b) shows no sign of healing. The tooth was followed for more than 5 years after endodontic re-treatment; incomplete healing was observed (c).
Endodontic re-treatment
When the tooth had a combined post, core, and crown, the entire unit was removed with a crown remover (Auto Abdicator, Alex Dental, Sweden) where possible. In other cases, the crown had to be removed before the post could be extracted with a post remover (JS Sjödings, Kista, Sweden). Rubber dam was then applied, and an aseptic technique was used throughout the endodontic treatment. The tooth, clamp, and surrounding parts of the rubber dam were cleaned with 30% hydrogen peroxide and then swabbed with 5% iodine tincture.13 After the tincture had dried, the tooth surface was swabbed with 5% sodium thiosulphate solution to inactivate the iodine tincture so that remnants of iodine would not influence the bacteriologic sampling.13 The root filling was removed by instrumentation with burs and files and without the use of chemical solvents. Measurement radiographs were taken to determine the canal length and to ensure that all guttapercha had been removed. Sterile saline solution was then introduced into the canal by syringe; this was done carefully so that the canal would not be overfilled. The canal was then instrumented so that material could be obtained from the walls. An initial bacteiologic sample was taken from the root canal by soaking up the fluid with charcoaled paper points. The root canals were left empty to allow any surviving bacteria in the root canal to multiply to a level that would be detectable at a subsequent appointment. The cavities were sealed with sterile foam pellets (3M, St Paul, Minn.) and zinc oxide-eugenol cement at least 4 mm deep.
At a second visit 7 days later, bacteriologic samples were taken after removal of the temporary filling. The root canals were then instrumented by means of hand files and irrigation with 0.5% sodium hypochlorite solution. After final irrigation the root canals were filled with calcium hydroxide paste (Calasept; Scania Dental AB, Knivsta, Sweden) by means of a spiral paste-filler. The access cavities were sealed with zinc oxide-eugenol cement at least 4 mm thick.
At a third appointment 7 to 14 days later, the dressing was rinsed out of the canals with sterile saline solution. The canal was lightly filed to remove loose calcium hydroxide remnants, and a postmedication sample was taken. The root canals were filled with gutta-percha by means of the lateral condensation technique. A master cone was adapted to the canal by dipping it in rosinchloroform, and then multiple accessory cones were laterally condensed after having been softened in chloroform. A zinc oxide-eugenol plug with a thickness of 1 mm to 2 mm was applied over the root filling. Subsequent restoration of the tooth was performed without rubber dam isolation and with a nonaseptic technique.
Bacteriologic sampling
Bacteriologic samples were obtained from the root canals in a procedure similar to that described previously.22-25 The initial sample, taken at the first appointment after the previous root filling had been removed, was taken in a liquid thioglycolate medium (11260; Baltimore Biological Laboratories, Cockeysville, Md.) supplemented with agar to prevent oxygen diffusion.26 This medium is highly effective in reducing oxygen so that toxic intermediates of oxygen do not accumulate even if the medium is exposed to oxygen for a short while.27
At the second appointment three samples were taken from each canal. Two samples were taken in peptone yeast extract glucose broth (PYG)28 and one sample in the agar-supplemented thioglycolate medium. Precautions were taken to avoid oxygen contamination of the PYG broth.29 At the third appointment the sample taken before root filling was deposited in the agarsupplemented thioglycolate medium.
Microbiologic examination of the samples
All samples were introduced into an anaerobic box with an atmosphere of 10% hydrogen and 5% carbon dioxide in nitrogen. One of the PYG broth tubes was agitated in a mechanical mixer until the paper points disintegrated, and tenfold serial dilutions were made in buffered salt solution.28 Aliquots from the PYG broth and from each of the dilutions were inoculated onto blood agar plates.28 The plates were incubated in the box at 37° C for at least 10 days and were observed daily for growth. If no growth had occurred after 1 week, new blood agar plates were inoculated from the other PYG broth. A root canal sample was considered to be free of living bacteria when no growth was observed on the plates inoculated from the PYG broth tubes and in the agar-supplemented thioglycolate medium.
When growth occurred, different colony morphotypes on the blood agar plates were isolated. The bacterial isolates were identified according to standard methods.28,30-34 The identity of some strains was confirmed by comparison of the mobility of their soluble proteins in sodium dodecyl sulphate-polyacrylamide gel electrophoresis35 to that of reference strains.
Follow-up examination
Patients were recalled yearly for clinical and radiographic examination. At the recall appointments the type of restoration and any clinical signs or symptoms associated with the teeth were recorded. Radiographs were taken with the same x-ray unit by means of the long cone technique and a standardized exposure and processing to obtain optimal diagnostic quality of the radiographs. Strindberg’s criteria were used to judge the success rate of the therapy.1 Briefly, treatment was considered successful when either the contours, width, and structure of the periodontal margin were normal or the periodontal contours were widened mainly around an excess of filling material. All cases in which those criteria were not fulfilled were judged as unsuccessful. The cases were followed for 5 years if complete healing had not taken place earlier. Radiographs were analyzed separately by two trained independent observers using a light-box with variable illumination and a magnification viewer.36 When there was disagreement, the two observers discussed the case in an effort to come to a consensus. If the observers still disagreed on a particular case, the opinion of a third specialist was taken as final.
Statistical analysis
A two-tailed t-test was used to calculate the correlation between initial size of the lesions and outcome of the treatment. Fisher’s exact test (one-tailed) was used when the influence of infection on the outcome of treatment was calculated.
RESULTS
The bacterial species recovered from 24 of 54 canals after removal of the previous root filling are presented in Table I. In 20 cases there was growth from both the sample taken at the initial appointment and the sample taken at the second appointment. In one case bacteria (Peptostreptococcus micros and Fusobacterium nucleatum) grew from the sample taken at the first appointment but not from the samples taken at the second appointment. In three cases microorganisms were recovered from the samples taken at the second appointment, after the root canals had been left empty but not in the initial sample. The microorganisms isolated from these three root canals were Streptococcus intermedius, Streptococcus parasanguis, Lactobacillus catenaforme, and Candida albicans.
In 19 cases a single species was present, in 4 cases there were two species present (S. intermedius and L. catenaforme, Eubacterium alactolyticum and Propionibacterium acnes, P. micros and Streptococcus mitis, P. micros and F. nucleatum), and in one case there was a polymicrobial infection consisting of four species (Streptococcus anginosus, Eubacterium timidum, Propionibacterium propionicum, and Bacteroides gracilis). In all nine cases in which Enterococcus faecalis was isolated, it was the only microorganism present in the canal.
Fifty of the 54 treated cases (93%) were available for recall. Thirty-seven of the lesions healed completely and 13 cases were judged as failures—a success rate of 74%. A successfully treated case is shown in Fig. 1, and an example of a case where the periapical lesion persisted is shown in Fig. 2.
Eighteen of 24 teeth (75%) from which bacteria were isolated after removal of the root filling healed completely, and 19 of the 26 teeth from which there were no cultivable microorganisms healed—a success rate of 73%. The difference between the outcomes for the two groups was not statistically significant. The success rate for the teeth from which E. faecalis was isolated after removal of the earlier root filling was somewhat lower (66%) than the average for the whole material.
In samples taken at the time of root filling, microorganisms were recovered from six root canals. Four of the lesions associated with these teeth did not heal. Three of these teeth contained bacteria of the species E. faecalis and the fourth root canal harboured Actinomyces israelii. In the two teeth in which there was healing of the periapical lesion, strains of E. faecalis had been isolated at the time of root filling. Of the teeth with no recoverable microorganisms at the time of root filling, 35 of 44 teeth healed—a success rate of 80%. Although there were a small number of teeth that contained bacteria at the time of root filling, there was a statistically significant difference (p = 0.033) in success rates between the teeth that had yielded positive bacteriologic samples at the time of root filling (33%) and those that had not (80%).
Table I. Microorganisms recovered from canals after removal of root filling
| Microbial species | No. of cases |
| Enterococcus faecalis | 9 |
| Streptococcus anginosus | 2 |
| Streptococcus constellatus | 1 |
| Streptococcus intermedius | 1 |
| Streptococcus mitis | 1 |
| Streptococcus parasanguis | 1 |
| Peptostreptococcus micros | 2 |
| Actinomyces israelii | 3 |
| Pseudoramibacter alactolyticus* | 1 |
| Eubacterium timidum | 1 |
| Lactobacillus catenaforme | 1 |
| Propionibacterium acnes | 1 |
| Propionibacterium propionicum | 1 |
| Fusobacterium nucleatum | 1 |
| Bacteroides gracilis | 3 |
| Candida albicans | 2 |
| *Formerly Eubacterium alactolyticum. |
The initial size of the periapical lesions appeared to have an influence on the outcome of treatment. The mean size of all initial lesions was 4.2 mm (range, 2–13 mm). The initial mean size of the lesions that healed was 3.7 mm (range, 2–6.5 mm); the initial mean size of the lesions that persisted was 5.6 mm (range, 2.5–13 mm). The difference in size between the lesions that healed and those that did not heal was statistically significant at p = 0.034.
Of the 50 cases that could be followed-up, most (37) were filled within 0.5 to 2 mm from the radiographic apex. Nine were filled flush with the apex, and four had root-filling excesses of less than 1 mm. There was no statistical difference in outcome with respect to the root-filling level.
DISCUSSION
Most cases of endodontic failure are thought to involve a continuing infection of the root canal system that results in a chronic periapical lesion after treatment, yet few studies have evaluated the bacteria associated with failed endodontic treatment. This study used advanced bacteriologic techniques on previously root-filled teeth to determine which microorganisms are associated with endodontic failures. The outcome of endodontic re-treatment was evaluated by long-term follow-up. This work has shown that the nature of the infection in previously treated cases differs markedly from that in untreated cases. It has also confirmed that a lower success rate applies to teeth that have undergone re-treatment than to untreated teeth with apical periodontitis. Two factors that were shown to have a negative influence on the prognosis are the presence of infection at the time of root filling and the size of the periapical lesion.
Table II. Success rates for nonsurgical endodontic retreatment
| Follow-up | Success rate | |
| Study (year) | period (yr) | (%) |
| Strindberg1 (1956) | 4 | 66* |
| 7 | 84* | |
| Grahnén and Hansson2 (1961) | 5 | 65* |
| Engström et al.37 (1964) | 4 | 74* |
| Molven6 (1976) | 3.5 | 64† |
| Molven and Halse7 (1988) | 10–17 | 7l† |
| Bergenholtz et al.18 (1979) | 2 | 48† |
| Allen et al.19 (1989) | 0.5–1.0 | 73‡ |
| Sjögren et al.8 (1990) | 8–10 | 62† |
| van Nieuwenhuysen et al.20 (1994) | 0.5–2.0 | 72 |
| Sundqvist et al. (this study) | 5 | 74† |
*Includes pulpotomized teeth, with and without periapical lesions.
†Only root-filled teeth with periapical lesions.
‡Excludes cases treated by surgery.
Seventy-four percent of cases healed after endodontic re-treatment. This figure compares favorably with previous follow-up studies of non-surgical re-treatment of root-filled teeth with periapical lesions (Table II). It should be noted that some studies that have reported similar or higher success rates included cases of retreatment of pulpotomized teeth without lesions1,2,37 or had follow-up periods too short to have allowed the repair process to stabilize.19,20 In this and earlier studies8,38 the periapical healing was rather slow in some cases; a follow-up period of at least 4 to 5 years was necessary for full reconstitution of the periodontal contour. Strindberg1 noticed that some cases actually healed in the period between a 4-year follow-up and a later check at 7 years (Table II).
There is surprisingly limited information on the microorganisms that can persist in the root canal following obturation. In this and previous studies13,14 the microbial flora detected in root-filled canals could be characterized as monoinfections of predominantly gram-positive microorganisms, with approximately equal proportions of facultative and obligate anaerobes (Table III). This composition differs markedly from that seen in infections of the untreated canal, which typically have a polymicrobial flora with approximately equal proportions of gram-negative and gram-positive bacteria and are dominated by obligate anaerobes.15-17,22-24 While selective pressures exist in the untreated canal that favor the establishment of a very restricted group of the oral flora,17,39 special conditions must also apply to microorganisms that can persist in the root-filled canal. These include the capacity of some microorganisms to withstand antibacterial measures during cleaning and medication and to survive in an environment in which there are scant available nutrients and in which cooperative relationships with other bacteria are minimal. Very few bacteria appear to have such a capacity. Although the bacteriologic conditions of the previous endodontic therapy were in most cases not known, in all but one case the treatment appeared to have reached a radiographically reasonable standard. The number of species isolated from re-treatment cases is probably contingent on the quality of the initial endodontic treatment. Teeth with poor treatment are more likely to have a flora similar to that found in untreated canals than are teeth with apparently well-cleaned canals, and teeth with poor treatment are also more likely to contain a greater number of species. In the present study one case was included in which the previous root filling was of very low quality; this was the only canal that contained a polymicrobial flora.
Table III. Bacteriologic findings in root-filled teeth with periapical lesions
| Molander | Sundqvist | ||
| Möller13 | et al.14 | et al. | |
| Finding | (1966) | (1994) | (this study) |
| Bacterial species per | |||
| root canal with bacteria | 1.6 | 1.7 | 1.3 |
| Anaerobic bacteria* | 51 | 39 | 42 |
| Gram-positive bacteria* | 80 | ND | 87 |
| E. faecalis† | 29 | 46 | 38 |
ND, Value not given.
*Percent of isolated bacteria.
†Percent of canals with bacteria
The common recovery of E. faecalis from the root canals of teeth in which the previous treatment has failed is notable. Enterococci are not favored by the conditions in the untreated canal, and when present they usually make up a very small proportion of the initial flora in the root canal.17,24,40-42 E. faecalis appears to be highly resistant to the medicaments used during treatment and is one of the few microorganisms that has been shown in vitro to resist the antibacterial effect of calcium hydroxide.24,43-46 Enterococci have also been shown to have an ability to survive in root canals as single organisms without the support of other bacteria.40 In this study, E. faecalis was isolated in 38% of teeth that had recoverable microorganisms, which suggests that it is an important agent in endodontic failure. The fact that E. faecalis is not normally present or is present in very low numbers in an untreated case indicates that E. faecalis can enter the canal, survive the antibacterial treatment, and then persist after obturation. A recent study47 found a higher proportion of E. faecalis in teeth whose canals lacked an adequate seal for a period during the treatment or were treated over 10 or more visits, which is consistent with earlier suggestions that E. faecalis usually enters the canal during treatment.48 When E. faecalis is present in low numbers initially, it can usually be eliminated; however, once established in the root canal system it is a difficult organism to eradicate. Another microorganism, Candida albicans, was present in two of the previously root-filled teeth. Although fungi have occasionally been reported in untreated cases,49 they have been found in cases in which treatment had been protracted,50 and they have been associated with endodontic failures.12 Like bacteria of the species E. faecalis, these microorganisms appear to have an ability to utilize opportunities created by the removal of other microbes and also to have the capacity to grow in the low-nutrient environment of the treated canal.
Nineteen of the 26 cases in which no microorganisms were isolated from the previously root-filled teeth healed after re-treatment. The most plausible explanation for the healing observed in these cases is that microorganisms were present and had maintained the periapical lesion, and that the infection had been managed by the antimicrobial measures applied during retreatment. Because no microorganisms were detected in the samples, this raises the question of whether the bacteriologic methods have been reliable. Although it is possible that some microorganisms could have been lost, the sampling and cultivation techniques used in this study have previously been shown to be highly effective in the isolation and recovery of extremely oxygen-sensitive bacteria.15,26,51 If the number of microorganisms was very low, then they may not have been recovered by the sampling technique. However, a more likely explanation is that microorganisms, while present, were inaccessible to sampling. Microbes may hide in anatomic branches of the root canal system or in apical areas that may have been obliterated during the previous treatment. Furthermore it cannot be ruled out that the microorganisms might have been eliminated when the previous root filling was removed, even though this was done mechanically and without solvents. Bacteriologic sampling from previously filled root canals is certainly difficult under these circumstances, but there is no reason to suspect that the microorganisms isolated in this and similar studies13,14 are unrepresentative of the flora in root-filled teeth with persisting periapical lesions.
The presence of infection at the time of re-obturation significantly increased the success rate of teeth undergoing re-treatment. Teeth that had positive bacteriologic samples at the time of root filling had a success rate of just 33%, whereas those that had negative samples had a success rate of 80%. Although there was a small number of teeth in the groups, this observation is consistent with the findings of a recent study52 that showed a 26% lower success rate for teeth that were infected at the time of obturation. This finding emphasizes the importance of targeting the re-treatment to the total elimination of microorganisms from the root canal system.
Long-term follow-up of the teeth revealed that the outcome of treatment was influenced by the size of the periapical lesions before re-treatment. Teeth with larger lesions had a poorer prognosis than teeth with smaller lesions; a similar tendency has been reported in other studies,20,37 although their material was insufficient for statistical evaluation. This finding stands in contrast to observations in untreated teeth in which the preoperative size of the lesions had no influence on the outcome of endodontic treatment.8 The likelihood that a lesion is a cyst increases with the size of the lesion53,54; there is a possibility that if some of the lesions were radicular cysts, this could have influenced the re-treatment outcome.
Conceivably, the two variables that were shown here to influence treatment outcome—infection at the time of root filling and initial size of the lesion—may be linked or may influence one another. However, statistical analysis showed no correlation between bacteria at the time of root filling and size of the periapical lesion, regardless of whether all cases or just the failure cases were considered. This indicates that the two variables influence the outcome separately and are independent of one another.
In a previous study12 histologic analysis of tissue specimens from periapical lesions refractory to conventional endodontic therapy revealed that the major cause of root canal treatment failures was microorganisms persisting in the apical parts of root canals of root-filled teeth. Bacteria can also prevent the healing of periapical lesions by establishing themselves in the periapical tissue. Two species, A. israelii and Propionibacterium propionicum, are known to have the ability to establish such extraradicular infections.9,55,56 In the present study, samples taken at the time of root filling revealed that one of the root canals harbored A. israelii at the time of root filling. The lesion associated with this tooth did not heal, probably because of a persistence of A. israelii in the periapical tissues.
The results of the present study show that three of four cases with previous root fillings and persisting periapical lesions can be successfully managed by conservative endodontic re-treatment. One in four lesions did not heal after re-treatment, and it is not known whether the cause was a persisting infection that might have been inaccessible to instrumentation. Extraradicular infections, cysts, and extruded filling materials that cause foreign-body reactions are additional factors that can adversely affect periapical healing after conventional endodontic re-treatment.10,11,57 The importance of conservative re-treatment of canals before surgery has been illustrated in a study58 that showed a re-treatment success rate 24% higher in cases of failed endodontic treatment in which antibacterial measures and refilling of the canal preceded apical surgery than in cases in which apical surgery was the only procedure performed. These results, in conjunction with the outcomes obtained in the present study, suggest that it is possible to attain very high success rates when both the intraradicular and the extraradicular causes of failure of endodontic treatment are well managed. The high success rate obtained in the present study suggests that defective root fillings should, when possible, be conventionally re-treated and then supplemented, if no signs of healing are apparent, with apical surgery.
We thank Mrs. Lilian Palmquist and Mrs. Sonia Andersson for their excellent clinical assistance.
REFERENCES
1. Strindberg LZ. The dependence of the results of pulp therapy on certain factors: an analytical study based on radiographic and clinical follow-up examinations. Acta Odont Scand 1956; 14:(supplement 21): 1-175.
2. Grahnén H, Hansson L. The prognosis of pulp and root canal therapy: a clinical and radiographic follow-up examination. Odontol Revy 1961;12:146-65.
3. Seltzer S, Bender IB, Turkenkopf S. Factors affecting successful repair after root canal therapy. J Am Dent Assoc 1963;67:651-62.
4. Storms JL. Factors that influence the success of endodontic treatment. J Can Dent Assoc 1969;35:83-97.
5. Kerekes K, Tronstad L. Long-term results of endodontic treatment performed with a standardized technique. J Endod 1979; 5:83-90.
6. Molven O. The frequency, technical standard and results of endodontic therapy. Norske Tannlaegeforenings Tidende 1976;86:142-7.
7. Molven O, Halse A. Success rates for gutta-percha and Kloroperka N-Ø root fillings made by undergraduate students: radiographic findings after 10-17 years. Int Endod J 1988;21: 243-50.
8. Sjögren U, Hägglund B, Sundqvist G, Wing K. Factors affecting the long-term results of endodontic treatment. J Endod 1990;16: 498-504.
9. Sjögren U, Happonen RP, Kahnberg KE, Sundqvist G. Survival of Arachnia propionica in periapical tissue. Int End J 1988;21: 277-82.
10. Nair PNR, Sjögren U, Krey G, Sundqvist G. Therapy-resistant foreign body giant cell granuloma at the periapex of a root-filled human tooth. J Endod 1990;16:589-95.
11. Nair PNR, Sjögren U, Schumacher E, Sundqvist G. Radicular cyst affecting a root-filled human tooth: a long-term post-treatment follow-up. Int End J 1993;26:225-33.
12. Nair PNR, Sjögren U, Kahnberg K-E, Sundqvist G. Intraradicular bacteria and fungi in root-filled asymptomatic human teeth with therapy-resistant periapical lesions: a longterm light and electron microscopic follow-up study. J Endod 1990;16:580-8.
13. Möller ÅJR. Microbiological examination of root canals and periapical tissues of human teeth. Odontologisk Tidskrift 1966;74:(supplement): 1-380.
14. Molander A, Reit C, Dahlén G, Kvist T. Microbial examination of root filled teeth with apical periodontitis [Abstract]. Int End J 1994;27:104.
15. Sundqvist G. Bacteriological studies of necrotic dental pulps. Umeå, Sweden: University of Umeå; 1976. Dissertation.
16. Wasfy MO, McMahon KT, Minah GE, Falkler WA. Microbial evaluation of periapical infections in Egypt. Oral Microbiol Immunol 1992;7:100-5.
17. Sundqvist G. Associations between microbial species in dental root canal infections. Oral Microbiol Immunol 1992;7:257-62.
18. Bergenholtz G, Lekholm U, Milthon R, Heden G, Ödesjö B, Engström B. Re-treatment of endodontic fillings. Scand J Dent Res 1979;87:217-24.
19. Allen RK, Newton CW, Brown CE. A statistical analysis of surgical and nonsurgical endodontic re-treatment cases. J Endod 1989;15:261-6.
20. van Nieuwenhuysen J-P, Aouar M, D’Hoore W. Re-treatment or radiographic monitoring in endodontics. Int Endod J 1994;27: 75-81.
21. Eggen S. Röntgenografiske tannmålinger i daglig praksis ved hjälp av standardisert parallell-teknikk og en kalibrert målelinjal. Tandläkartidningen 1974;66:10-2.
22. Byström A, Sundqvist G. Bacteriological evaluation of the efficacy of mechanical root canal instrumentation in endodontic therapy. Scand J Dent Res 1981;89:321-8.
23. Byström A, Sundqvist G. Bacteriologic evaluation of the effect of 0.5 per cent sodium hypochlorite in endodontic therapy. Oral Surg Oral Med Oral Pathol 1983;55:307-12.
24. Byström A, Sundqvist G. The antibacterial action of sodium hypochlorite and EDTA in 60 cases of endodontic therapy. Int Endod J 1985;18:35-40.
25. Sjögren U, Sundqvist G. Bacteriologic evaluation of ultrasonic root canal instrumentation. Oral Surg Oral Med Oral Pathol 1978;63:366-70.
26. Carlsson J, Sundqvist G. Evaluation of methods of transportation and cultivation of bacterial specimens from infected root Canals. Oral Surg Oral Med Oral Pathol 1980;49:451-4.
27. Carlsson J, Nyberg G, Wrethén J. Hydrogen peroxide and superoxide radical formation in anaerobic broth media exposed to atmospheric oxygen. Applied and Environmental Microbiology 1978;36:223-9.
28. Holdeman LV, Cato EP, Moore WEC. Anaerobe laboratory manual. 4th ed. Blacksburg, VA: Virginia Polytechnic Institute and State University; 1977.
29. Fulghum RS. Mobile anaerobe laboratory. Applied Microbiology 1971;21:769-70.
30. Cowan ST. Cowan and Steel’s manual for the identification of medical bacteria. 2nd ed. Cambridge: Cambridge University Press; 1974.
31. Krieg NR, Holt JG, editors. Bergey’s manual of systematic bacteriology. Vol. 1. Baltimore: Williams & Wilkins; 1984.
32. Sneat PHA, Mair NS, Sharpe ME, Holt JG, editors. Bergey’s manual of systematic bacteriology. Vol. 2. Baltimore: Williams & Wilkins; 1986.
33. Hill GH, Ayers OA, Kohan AP. Characteristics and sites of infection of Eubacterium nodatum, Eubacterium timidum, Eubacterium brachy, and other asaccharolytic eubacteria. J Clin Microbiol 1987;25:1540-5.
34. Willems A, Collins MD. Phylogenetic relationships of the genera Acetobacterium and Eubacterium sensu stricto and reclassification of Eubacterium alactolyticum as Pseudoramibacter alactolyticus gen. nov., comb. nov. International Journal of Systematic Bacteriology 1996;46:1083-7.
35. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680-5.
36. Halse A, Molven O. A strategy for the diagnosis of periapical pathosis. J Endod 1986;12:534-8.
37. Engström B, Hård af Segerstad L, Ramström G, Frostell G. Correlation of positive cultures with the prognosis for root canal treatment. Odontol Rev 1964;15:257-70.
38. Byström A, Happonen R-P, Sjögren U, Sundqvist G. Healing of periapical lesions of pulpless teeth after endodontic treatment with controlled asepsis. Endod Dent Traumatol 1987;3:58-63.
39. Sundqvist G, Figdor D. Endodontic treatment of apical periodontitis. In: Ørstavik D, Pitt Ford T, editors. Essential endodontology. Oxford: Blackwell Science Ltd; 1998.
40. Fabricius L, Dahlén G, Holm SE, Möller ÅJR. Influence of combinations of oral bacteria on periapical tissues of monkeys. Scand J Dent Res 1982;90:200-6.
41. Sato T, Hoshino E, Uematsu H, Noda T. Predominant obligate anaerobes in necrotic pulps of human deciduous teeth. Microbial Ecology in Health and Disease 1993;6:269-75.
42. Weiger R, Manncke B, Werner H, Löst C. Microbial flora of sinus tracts and root canals of non-vital teeth. Endod Dent Traumatol 1995;11:15-9.
43. Stevens RH, Grossman LI. Evaluation of the antimicrobial potential of calcium hydroxide as an intracanal medicament. J Endod 1983;9:372-4.
44. Haapasalo M, Ørstavik D. In vitro infection and disinfection of dentinal tubules. J Dent Res 1987;66:1375-9.
45. Ørstavik D, Haapasalo M. Disinfection by endodontic irrigants and dressings of experimentally infected dentinal tubules. Endod Dent Traumatol 1990;6:142-9.
46. Safavi KE, Spångberg LSW, Langeland K. Root canal dentinal tubule disinfection. J Endod 1990;16:207-10.
47. Siren EK, Haapasalo MPP, Ranta K, Salmi P, Kerosuo ENJ. Microbiological findings and clinical treatment procedures in endodontic cases selected for microbiological investigation. Int Endod J 1997;30:91-5.
48. Sjögren U, Figdor D, Spångberg L, Sundqvist G. The antimicrobial effect of calcium hydroxide as a short-term intracanal dressing. Int Endod J 1991;24:119-25.
49. Sen BH, Piskin B, Demirci T. Observation of bacteria and fungi in infected root canals and dentinal tubules by SEM. Endod Dent Traumatol 1995;11:6-9.
50. Waltimo TMT, Siren EK, Torkko HLK, Olsen I, Haapasalo MPP. Fungi in therapy-resistant apical periodontitis. Int Endod J 1997;30:96-101.
51. Carlsson J, Frölander F, Sundqvist G. Oxygen tolerance of anaerobic bacteria isolated from necrotic dental pulps. Acta Odontol Scand 1977;35:139-45.
52. Sjögren U, Figdor D, Persson S, Sundqvist G. Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis. Int Endod J 1997;30:297-306.
53. Lalonde ER. A new rationale for the management of periapical granulomas and cysts:an evaluation of histopathological and radiographic findings. J Am Dent Assoc 1970;80:1056-9.
54. Natkin E, Oswald RJ, Carnes LI. The relationship of lesion size to diagnosis, incidence and treatment of periapical cysts and granulomas. Oral Surg Oral Med Oral Pathol 1984;57:82-94.
55. Sundqvist G, Reuterwing C-O. Isolation of Actinomyces israelii from periapical lesion. J Endod 1980;6:602-6.
56. Nair PNR, Schroeder H E. Periapical actinomycosis. J Endod 1984;10:567-70.
57. Nair PNR, Pajarola G, Schroeder HE. Types and incidence of human periapical lesions obtained with extracted Teeth. Oral Surg Oral Med Oral Pathol 1996;81:93-102.
58. Grung B, Molven O, Halse A. Periapical surgery in a Norwegian county hospital: follow-up findings of 477 teeth. J Endod 1990;16:411-7.
Reprint requests:
G. Sundqvist, DDS, PhD
Department of
Endodontics
Faculty of Odontology
Umeå University
90187
Umeå
Sweden
aProfessor and Head of Endodontics Department, Umeå University.
bSpecialist in Endodontics, private practice; Lecturer, School of Dental Science, University of Melbourne.
cAssociate Professor, Department of Oral and Maxillofacial Surgery, Umeå University.
dAssociate Professor, Department of Endodontics, Umeå University.
Received for publication July 17, 1997; accepted for publication Aug. 22, 1997.
Copyright © 1998 by Mosby, Inc.
079-2104/98/$5.00 + 0 7/15/86295