International Endodontic Journal (1991) 24, 119–125
The antimicrobial effect of calcium hydroxide as a short-term intracanal dressing
U. SJÖGREN, D. FIGDOR*, L. SPÅNGBERG† & G. SUNDQVIST Department of Endodontics, University of Umeå, Umeå, Sweden, *Melbourne, Australia and †Department of Endodontology, University of Connecticut, Farmington, Connecticut, USA
Summary. The antibacterial effect of calcium hydroxide as a short-term intracanal dressing was clinically evaluated by applying the medicament for 10 minutes or 7 days in root canals of teeth with periapical lesions. The results showed that the 7-day dressing efficiently eliminated bacteria which survived biomechanical instrumentation of the canal, while the 10-minute application was ineffective.
Introduction
It has been established beyond doubt that bacteria play a decisive role in the development of apical periodontitis (Kakehashi et al. 1965, Sundqvist 1976, Möller et al. 1981). Consequently, one of the major goals of endodontic treatment is the elimination of all bacteria from the root canal (Grossman 1981, Schilder 1984). This is normally accomplished by mechanical instrumentation supported by various irrigating solutions, and antibacterial dressing of the canal between appointments.
Mechanical instrumentation and irrigation with antibacterial solutions have been considered essential for the elimination of bacteria during endodontic treatment, whereas the need for intracanal dressings has been questioned (Strindberg 1965, Schilder 1984, Weine 1989). Results of our previous studies support the view that irrigation and mechanical cleansing greatly reduce the number of bacteria in the root canal. However, half of the treated root canals still harboured bacteria after one treatment with careful mechanical instrumentation and the use of an antimicrobial irrigating solution (Byström & Sundqvist 1981, 1983, 1985). Thus, more intensive treatment of the root canal with an antibacterial substance must still remain an important adjunct in the total elimination of bacteria during endodontic treatment.
Recent results show that calcium hydroxide paste used as a dressing in carefully instrumented and irrigated root canals kills the bacteria so effectively that the treatment of initially infected root canals can be completed at the second visit (Byström et al. 1985). Calcium hydroxide maintains its antibacterial effect over a long period of time, due to the slow release of hydroxyl ions (Proell 1949). In studies that have demonstrated the antimicrobial efficacy of calcium hydroxide, the root canals had been dressed for at least 1 month (Cvek et al. 1976, Byström et al. 1985). It is not clear, however, what minimum time might be needed for a calcium hydroxide dressing to achieve an optimal antibacterial effect. In vitro experiments have shown that many commonly occurring bacteria of the normal root canal flora are rapidly killed when exposed to calcium hydroxide for periods of 1–6 minutes (Byström et al. 1985).
The aim of the present study was to evaluate the antibacterial effectiveness of calcium hydroxide when used as a short-term intracanal dressing in vivo.
Material and methods
Clinical material
Thirty teeth were used, all of which had single roots containing necrotic pulps, intact pulp chamber walls, and radiographic evidence of periapical bone lesions, which were referred for treatment during a limited time period. Radiographic examination was carried out by the paralleling technique with Kodak film (Ultraspeed1) (24 × 36 mm) in a film holder (Eggen 1974).
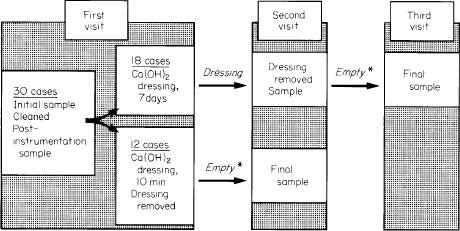
Fig. 1. Diagram showing study design. *Canals sealed, with no dressing. Four out of 12 cases dressed for 10 minutes and 14 out of 18 cases dressed for 7 days were left empty for 1·5 to 5 weeks; all other cases were left empty for 1 week.
Treatment
Rubber dam and an aseptic technique were used throughout treatment. After access had been gained to the root canal, the working length was determined. During this process, care was taken not to penetrate the apical foramen. Narrow root canals were initially enlarged by hand filing with Hedstrom files until a size 20 file could be introduced to the working length. They were then filed ultra-sonically2–4 for approximately 3 minutes. Wider root canals were initially treated with ultrasonic preparation. Endosonic files were used within 1–2 mm of the working length. Hand files were used to shape the apical portion of the canal. The canals were instrumented to a size 40 Hedstrom or larger at the working length. Sodium hypochlorite (0.5 per cent) was used as an irrigant. After final irrigation the root canals were carefully dried with paper points and filled with a calcium hydroxide paste5 by means of a lentulo spiral filler and packed with the blunt end of a paper point. In 12 cases the calcium hydroxide paste was left in the canal for 10 minutes and in 18 cases the calcium hydroxide paste was left in the canal as a dressing for 7 days (Fig. 1). Sterile foam pellets6 were placed in the chambers of teeth in which the canal had been left empty. The access cavities were, in all cases, sealed with at least 4 mm thickness of zinc oxide–eugenol cement.
Procedure for obtaining bacterial samples from the root canals
The procedure for obtaining samples from the canals was the same as used previously (Byström & Sundqvist 1981, 1983, 1985, Sjögren & Sundqvist 1987). Sterile saline solution was introduced into the canal by means of a syringe, and the canal was enlarged until a medium-sized paper point could be introduced to a level approximately 1 mm short of the radiographic apex. The fluid in the canal was absorbed with charcoaled paper points and transferred to a tube with 5 ml of anaerobic peptone yeast extract glucose (PYG) broth (Holdeman et al. 1977). Precautions were taken to avoid oxygen contamination of the PYG broth by means of a mobile anaerobe laboratory (Fulghum 1971). The canal was again filled with sampling fluid, and a second sample was taken in the same way. This sample was used as a control for the first sample.
After mechanical instrumentation and irrigation of the canal, any remaining sodium hypochlorite was inactivated by rinsing the canal with a 5 per cent sodium thiosulphate solution (Möller 1966). Subsequently, a post-instrumentation sample was taken from the canal.
Following the application of calcium hydroxide for 10 minutes or 7 days, dressings were rinsed out of the canal using sterile saline solution. The canal walls were filed lightly to remove loose calcium hydroxide remnants, and a postmedication sample was taken from the root canals which had been dressed for 7 days (Fig. 1). The canals were then sealed with sterile foam pellets and zinc oxide–eugenol cement, and left empty for 1 week (12 cases) or for extended periods of up to 5 weeks (18 cases), at which time a final sample was taken (Fig. 1).
Microbiological examination of the samples
The PYG broth tubes with the samples were introduced into an anaerobic box with an atmosphere of 10 per cent hydrogen and 5 per cent carbon dioxide in nitrogen. The tubes were agitated in a mechanical mixer until the paper points disintegrated, and tenfold serial dilutions to 103 were made in dilution blanks (Holdeman et al. 1977). Aliquots of 0.5 ml and 0.1 ml from the PYG broth, and aliquots of 0.1 ml from each of the dilutions were inoculated on to duplicate blood agar plates (Holdeman et al. 1977). In addition, one plate selective for enterococci (Sabbaj et al. 1971), and one plate selective for Actinobacillus actinomycetemcomitans (Slots 1982), and one Columbia agar plate were inoculated with 0.1 ml of the PYG broth. The Columbia agar contained (per litre) 42.5 g of Columbia agar base,1 6.5 g of Bactoagar2, 5 mg of haemin, 10 mg of menadione, and 50 ml of horse blood. The plate selective for A. actinomycetemcomitans was incubated for 7 days in 10 per cent CO2 in air at 37°C. One of the duplicate set of blood agar plates was incubated aerobically for 48 hours at 37°C; the other plate was incubated in the box at 37°C for at least 10 days. The latter plates were observed daily for growth. When no growth had occurred after 1 week, new blood agar plates were inoculated from the duplicate samples. These tubes had not been used for serial dilutions. This procedure was repeated after another week before the sample was considered to be free of living bacteria.
The total number of bacteria in all samples was determined. Bacteria in the initial samples were characterized to genus level and were then stored, frozen, for comparison with subsequent isolates from the root canal. Bacteria recovered from root canals after biomechanical cleaning and antibacterial dressing were identified to the species level. They were characterized as described by Holdeman et al. (1977), Hardie & Bowden (1976), Tanner et al. (1981), Schleifer & Kilpper-Bälz (1984), and van Winkelhoff et al. (1985).
Results
Bacteria were initially present in all 30 root canals. The median number of bacterial cells present in the initial samples from those root canals that were treated with calcium hydroxide for 10 minutes was 6.5 × 103 (range < 102–1 × 108). In those 18 root canals subsequently treated with calcium hydroxide dressing for 7 days, the median value of bacterial cells was initially 9.8 × 104 (range < 102–6.4 × 106).
After the root canals had been instrumented, irrigated and filed ultrasonically, bacteria were present in 6 of the 12 canals which were subsequently treated with calcium hydroxide for 10 minutes, and in 9 of the 18 canals dressed with calcium hydroxide for 7 days. The number of bacterial cells in these samples was usually less than 102 and only two samples contained more than 103 bacterial cells.
In the 18 canals which had been dressed for 1 week, bacteria were not found in samples taken immediately following removal of the dressing, nor could bacteria be recovered in samples taken 1–5 weeks later when the canals had been sealed without an antibacterial dressing. The 10-minute application of calcium hydroxide was not effective since bacteria persisted at the second appointment in 6 root canals (Table I). All of the persisting strains were present in the initial samples, except in one case (EQ). Bacteria were isolated after biomechanical treatment in three root canals, whilst the other three root canals gave negative samples after biomechanical treatment (Table I). In three further cases, bacteria which had survived biomechanical instrumentation were eliminated by the 10-minute application of calcium hydroxide (Table II). There was no relationship between an extended period (> 1 week) in which the canal was left empty, and recovery of bacteria. Of the six cases in which bacteria were present in the final sample, four were left empty for 1 week and two (KS and UP) remained empty for longer than 1 week.
Table I. Bacteria persisting after treatment for 10 minutes with calcium hydroxide dressing
| No. of bacteria in the sample | ||||
| Case | Initially | After biomechanical treatment | Second appointment* | Persisting strains |
| KS | 1 × 108 | <102 | 5 × 105 | Fusobacterium nucleatum Bacteroides denticola |
| UP | 6.5 × 103 | — | 7.5 × 105 | Fusobacterium nucleatum Fusobacterium species |
| AE | 1.5 × 106 | 2.7 × 102 | 0.3 × 102 | Fusobacterium nucleatum |
| EQ | 1.0 × 102 | <102 | 4.0 × 102 | Enterococcus faecalis |
| BOL | 1.9 × 103 | — | 5.0 × 105 | Actinomyces viscosus Bacteroides buccae Peptostreptococcus micros Wolinella recta |
| SB | 3.5 × 104 | — | 1.0 × 105 | Eubacterium alactolyticum Lactobacillus catenaforme Lactobacillus species Fusobacterium species |
*Second appointment after leaving the root canals empty and the access cavity closed for 1–5 weeks.
Table II. Bacteria eliminated by 10-minute application of calcium hydroxide dressing
| No. of bacteria in the sample | ||||
| Case | Initially | After biomechanical treatment | Second appointment | Strains persisting after biomechanical treatment |
| KH | 3.5 × 106 | <102 | — | Bacteroides species |
| HK | 5.5 × 103 | 2.6 × 102 | — | Lactobacillus species |
| GH | 6.5 × 103 | <102 | — | Peptostreptococcus micros |
Thirteen of the 14 strains isolated after calcium hydroxide application were anaerobic (Table I). The facultatively anaerobic isolate was an Enterococcus faecalis strain. Strains which could not be classified to the species level (Tables I and II) were anaerobic, non-motile, small non-pigmenting rods. They were identified as Fusobacterium, Bacteroides and Lactobacillus species in accordance with earlier studies (Byström & Sundqvist 1985, Sundqvist et al. 1989). Enterococcus faecalis was initially present in three cases treated with calcium hydroxide dressing for 7 days; in two cases these bacteria did not survive the bio-mechanical instrumentation, and in the third case the bacteria perished after dressing with calcium hydroxide.
Discussion
The application of calcium hydroxide has previously been shown to eliminate bacteria efficiently from the root canal when used as an intracanal dressing for 1 month (Cvek et al. 1976, Byström et al. 1985). In the present study calcium hydroxide was shown to be highly effective in killing the persisting root canal flora, when canals were dressed for 7 days. Root canals were left empty for 1–5 weeks after removal of calcium hydroxide to ensure that any bacteria which had survived, but were present only in low numbers after the antibacterial dressing, would be able to grow and be recovered by sampling. Bacteria were not recovered, however, at any interval after removal of the 7-day dressing, which confirmed that bacteria could not survive a 7-day application of calcium hydroxide.
It has previously been observed that many bacteria commonly present in the necrotic pulp are rapidly killed when exposed to a saturated solution of calcium hydroxide, in vitro, for 1–6 minutes (Byström et al. 1985). When calcium hydroxide was applied for 10 minutes in vivo, however, the material was ineffective in destroying bacteria which had persisted following biomechanical instrumentation since half of the canals still contained detectable bacteria. The inefficiency of the short application of calcium hydroxide may well be due to failure of the medicament to reach the intended site within 10 minutes. It has recently been demonstrated that hydroxyl ions do not readily diffuse through dentine due to the buffering capacity of hydroxyapatite (Wang & Hume 1988). Eventually this inherent buffering capacity can be overcome given sufficient availability of hydroxyl ions over time (Wang & Hume 1988). When calcium hydroxide is applied to the root canal for 1 month, a diffusion gradient develops for the hydroxyl ions, since pH values are higher centrally than at the periphery of the tooth root (Tronstad et al. 1981). Thus bacteria may be initially protected from exposure to lethal hydroxyl ions by the buffering capacity of the dentine in which the bacteria are situated. For example, E. faecalis, a species which is resistant to rapid killing by calcium hydroxide (Stevens & Grossman 1983, Byström et al. 1985), was recently shown to be eliminated from infected dentine specimens in vitro by prolonged exposure (24 hours) to calcium hydroxide, despite the fact that it survived shorter exposures (Safavi et al. 1990).
Calcium hydroxide is rather insoluble (Weast et al. 1984), and the release of hydroxyl ions, which are required for killing of bacteria (Proell 1949) is dependent on an aqueous environment. When calcium hydroxide is suspended in water, in vitro, there is an optimal release of hydroxyl ions; in the instrumented canal, however, the release of hydroxyl ions may be limited by a decline in the availability of water molecules. Furthermore, the destruction of bacteria in vitro was facilitated by an extensive surface area accessible to hydroxyl ions (Byström et al. 1985); this is unlikely to be the case clinically in the instrumented canal. Bacteria may occur as small colonies (Nair 1987) in which cells at the centre could be protected by cells lying at the periphery. Furthermore, micro-organisms lining dentinal tubules may be exposed to hydroxyl ions initially only at the tubule orifice. Apart from being located within dentinal tubules, bacteria may also be enclosed within anatomical variations of the root canal system, such as lateral canals and the reticulated network of pulp tissue which extends outward from the tooth centre. Bacteria may additionally be situated within pulp tissue remnants or smear layer which remains following canal instrumentation. Eventually, however, continued diffusion of hydroxyl ions will raise the pH sufficiently for the destruction of most or all of the root canal flora.
Enterococcus faecalis has been reported to be present in a higher number of final than initial root canal samples (Molander et al. 1990). Enterococcusfaecalis was present in three initial samples, and in each case it was eradicated during the biomechanical instrumentation or 7-day antibacterial dressing. Mechanical instrumentation was previously found to reduce the number of bacterial cells 1000-fold in the canal (Byström & Sundqvist 1981), and the use of antibacterial irrigants and ultrasonic filing increased the antibacterial effect even more (Byström & Sundqvist 1985, Sjögren & Sundqvist 1987). These measures are apparently sufficient for the elimination of E. faecalis when initially present in low numbers, since persistence of E. faecalis has not been a problem in the treatment of non-vital cases in this and earlier studies (Byström & Sundqvist 1981, 1983, 1985, Byström et al. 1985). However, the finding that enterococci are present more frequently in samples from vital cases (Mejare 1975) and in teeth with extensive coronal destruction (Engström et al. 1970) suggests that they may enter the root canal from carious lesions and the gingival sulcus which appear to be normal oral habitats of these micro-organisms (Gibbons et al. 1963, Engström 1964).
In summary, this work confirms earlier findings that a calcium hydroxide dressing efficiently eliminates bacteria which may survive biomechanical instrumentation, and that reliable, predictable results can be achieved by dressing the canal with calcium hydroxide for 7 days.
References
BYSTRÖM, A. & SUNDQVIST, G. (1981) Bacteriologic evaluation of the efficacy of mechanical root canal instrumentation in endodontic therapy. Scandinavian Journal of Dental Research, 89, 321–328.
BYSTRÖM A. & SUNDQVIST, G. (1983) Bacteriologic evaluation of the effect of 0.5 per cent sodium hypochlorite in endodontic therapy. Oral Surgery, Oral Medicine and Oral Pathology, 55, 307–312.
BYSTRÖM A. & SUNDQVIST, G. (1985) The antibacterial action of sodium hypochlorite and EDTA in 60 cases of endodontic therapy. International Endodontic Journal, 18, 35–40.
BYSTRÖM A., CLAESSON, R. & SUNDQVIST, G. (1985) The antibacterial effect of camphorated paramonochlorophenol, camphorated phenol and calcium hydroxide in the treatment of infected root canals. Endodontics and Dental Traumatology, 1, 170–175.
CVEK, M., HOLLENDER, L. & NORD, C.E. (1976) Treatment of non-vital permanent incisors with calcium hydroxide. Odontologisk Revy, 27, 93–108.
EGGEN, S. (1974) Röntgenografiske tannmålinger i daglig praksis ved hjelp av standardisert parallell-teknikk og en kalibrert målelinjal. Tandläkartidningen, 66, 10–12.
ENGSTRÖM, B. (1964) The significance of enterococci in root canal treatment. Odontologisk Revy, 15, 87–106.
ENGSTRÖM, B., POLHAGEN, L. & SPÅNGBERG, L. (1970) Rotkanalsinfektionens typ och frekvens. Odontologiska Samfundets i Finland Årsbok, pp. 7–18.
FULGHUM, R.S. (1971) Mobile anaerobe laboratory. Applied Microbiology, 21, 769–770.
GIBBONS, R.J., SOCRANSKY, S.S., KAPSIMALIS, B. & MACDONALD, J.B. (1963) The microbiota of the gingival crevice area of man—II. The predominant cultivable organisms. Archives of Oral Biology, 8, 281–289.
GROSSMAN, L.I. (1981) Endodontic Practice, 10th edn, p. 200. Lea & Febiger, Philadelphia.
HARDIE, J.M. & BOWDEN, G.H. (1976) Physiological classification of oral viridans streptococci. Journal of Dental Research, 55, A166–176.
HOLDEMAN, L.V., CATO, E.P. & MOORE, W.E. (1977) Anaerobe Laboratory Manual, 4th edn. Anaerobe Laboratory, Virginia Polytechnic Institute and State University, Blacksburg, Virginia.
KAKEHASHI, S., STANLEY, H.R. & FITZGERALD, R.J. (1965) The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surgery, Oral Medicine and Oral Pathology, 20, 340–349.
MEJARE, B. (1975) On the facultative anaerobic streptococci in infected dental root canals in man. Odontologisk Revy, 26 (Supplement 33), 1–26.
MOLANDER, A., REIT, C. & DAHLÉN, G. (1990) Microbiological evaluation of clindamycin as a root canal dressing in teeth with apical periodontitis. International Endodontic Journal, 23, 113–118.
MÖLLER, Å.J.R. (1966) Microbiological examination of root canals and periapical tissues of human teeth. Odontologisk Tidskrift, 74 (Supplement), 1–380.
MÖLLER, Å.J.R., FABRICIUS, L., DAHLÉN, G., ÖHMAN, A.E., HEYDEN, G. (1981) Influence on periapical tissues of indigenous oral bacteria and necrotic pulp tissue in monkeys. Scandinavian Journal of Dental Research, 89, 475–484.
NAIR, P.N.R. (1987) Light and electron microscopic studies of root canal flora and periapical lesions. Journal of Endodontics, 13, 29–39.
PROELL, F. (1949) Über die Eigenschaften des Calxyls und seine Vorzüge vor anderen in der zahnärztlichen Praxis angewandten Medikamenten. Zahnärtztliche Rundschau, 58, 255–259.
SABBAJ, J., SUTTER, V.L. & FINEGOLD, S.M. (1971) Comparison of selective media for isolation of presumptive group D streptococci from human faeces. Applied Microbiology, 22, 1008–1011.
SAFAVI, K.E., SPÅNGBERG, L.S.W. & LANGELAND, K. (1990) Root canal dentinal tubule disinfection. Journal of Endodontics, 16, 207–210.
SCHILDER, H. (1984) Canal debridement and disinfection. In Pathways of the Pulp. (eds, S. Cohen and R.C. Burns), 3rd edn, pp. 175–202. C.V. Mosby Co., St Louis.
SCHLEIFER, K.H. & KILPPER-BÄLZ, R. (1984) Transfer of Streptococcus faecalis and Streptococcus faecium to the genus Enterococcus nom. rev. as Enterococcus faecalis comb. nov. and Enterococcus faecium comb. nov. International Journal of Systemic Bacteriology, 31, 31–34.
SJöGREN, U. & SUNDQVIST, G. (1987) Bacteriologic evaluation of ultrasonic root canal instrumentation. Oral Surgery, Oral Medicine and Oral Pathology, 63, 366–370.
SLOTS, J. (1982) Selective medium for isolation of Actinobacillus actinomycetemcomitans. Journal of Clinical Microbiology, 15, 606–609.
STEVENS, R.H. & GROSSMAN, L.I. (1983) Evaluation of the antimicrobial potential of calcium hydroxide as an intracanal medicament. Journal of Endodontics, 9, 372–374.
STRINDBERG, L.Z. (1965) Det antibakteriella inläggets effekt vid konserverande rotbehandling. En jämförande bakteriologisk studie. Svensk Tandläkare Tidskrift, 58, 219–235.
SUNDQVIST, G. (1976) Bacteriological studies of necrotic dental pulps. Odontological Dissertation No. 7, University of Umeå, Sweden.
SUNDQVIST, G., JOHANSSON, E. & SJöGREN, U. (1989) Prevalence of black-pigmented Bacteroides species in root canal infections. Journal of Endodontics, 15, 13–19.
TANNER, A.C.R., BADGER, S., LAI, C.-H., LISTGARTEN, M.A., VISCONTI, R.A. & SOCRANSKY, S.S. (1981) Wolinella gen. nov., Wolinella succinogenes (Vibrio succinogenes Wolin et al.) comb. nov., and description of Bacteroides gracilis sp. nov., Wolinella recta sp. nov., Campylobacter concisus sp. nov., and Eikenella corrodens from humans with periodontal disease. International Journal of Systematic Bacteriology, 31, 432–445.
TRONSTAD, L., ANDREASEN, J.O., HASSELGREN, G., KRISTERSON, L. & RIIS, I. (1981) pH changes in dental tissues after root canal filling with calcium hydroxide. Journal of Endodontics, 7, 17–21.
VAN WINKELHOFF, A.J., VAN STEENBERGEN, T.J.M., KIPPUW, N. & DE GRAAFF, J. (1985) Further characterization of Bacteroides endodontalis, an asaccharolytic black-pigmented Bacteroides species from the oral cavity. Journal of Clinical Microbiology, 22, 75–79.
WANG, J.-D. & HUME, W.R. (1988) Diffusion of hydrogen ion and hydroxyl ion from various sources through dentine. International Endodontic Journal, 21, 17–26.
WEAST, R.C., ASTLE, M.J. & BEYER, W.H. (eds) (1984–1985) Handbook of Chemistry and Physics, 65th edn, p. B222. CRC Press Inc., Bocca Raton, Florida.
WEINE, F.S. (1989) Endodontic therapy. 4th edn, p. 351. C.V. Mosby Co., St Louis.
____________
Correspondence: Dr Ulf Sjögren, Department of Endodontics, Faculty of Odontology, University of Umeå, S-901 87 Umeå, Sweden.
1 Eastman Kodak Company, Rochester, New York, NY, USA.
2 Cavi-Endo, Dentsply International Inc., York, Pennsylvania, USA.
3 Enac, Osada Electric Co. Ltd, Tokyo, Japan.
4 Piezotec Satelec ZJ du Phare—33700 Mérignac, France.
5 Calasept, Scania Dental AB, Knivsta, Sweden.
6 3M Co., Dental Products Division, St Paul, MN, USA.
1 BBL Microbiology System, Cockeysville, MD, USA.
2 Difco Laboratories, Detroit, MI, USA.