International Endodontic Journal (1997) 30, 297–306
Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis
U. SJÖGRENa, D. FIGDORb, S. PERSSONa & G. SUNDQVISTa
aFaculty of Odontology, Umeå University, Sweden, and bMelbourne, Australia
Summary
This study investigated the role of infection on the prognosis of endodontic therapy by following-up teeth that had had their canals cleaned and obturated during a single appointment. The root canals of 55 single-rooted teeth with apical periodontitis were thoroughly instrumented and irrigated with sodium hypochlorite solution. Using advanced anaerobic bacteriological techniques, post-instrumentation samples were taken and the teeth were then root-filled during the same appointment. All teeth were initially infected; after instrumentation low numbers of bacteria were detected in 22 of 55 root canals. Periapical healing was followed-up for 5 years. Complete periapical healing occurred in 94% of cases that yielded a negative culture. Where the samples were positive prior to root filling, the success rate of treatment was just 68% – a statistically significant difference. Further investigation of three failures revealed the presence of Actinomyces species in each case; no other specific bacteria were implicated in failure cases. These findings emphasize the importance of completely eliminating bacteria from the root canal system before obturation. This objective cannot be reliably achieved in a one-visit treatment because it is not possible to eradicate all infection from the root canal without the support of an inter-appointment antimicrobial dressing.
Keywords: bacteria, one visit treatment, prognosis, root filling, success rate.
Introduction
Because bacteria are critical for the development of apical periodontitis (Kakehashi et al. 1965, Sundqvist 1976, Möller et al. 1981) modern endodontic treatment is directed at the elimination of microorganisms from the infected root canal before obturation. Follow-up studies that have examined the outcome of endodontic therapy have revealed a very high success rate when a negative bacterial culture was a prerequisite before root filling (Sjögren et al. 1990). In some circumstances bacteria can survive root canal preparation, and the long-term outcome of endodontic therapy is uncertain if bacteria are present in the root canal at the time of obturation.
Previous studies have shown that instrumentation and antibacterial irrigation with sodium hypochlorite solution will render about 50% of canals bacteria-free; the remaining canals contain small numbers of recoverable bacteria (Byström & Sundqvist 1985). When the biomechanical preparation is combined with an antimicrobial dressing applied to the clean canal for a suitable length of time before root filling, bacteria can be reliably eliminated from the canal (Byström et al. 1985, Sjögren et al. 1991). However, in some cases, such as when treatment is completed in one visit, no interappointment antimicrobial dressing is used and bacteria may be present in the canal at the time of root filling.
Only a few studies have evaluated the effect of infection at the time of root filling on the prognosis of treatment. These studies have shown that the success rate of endodontic treatment is approximately 10-15% lower for teeth which yield a positive culture before obturation than for teeth which yield a negative culture (Zeldow & Ingle 1963, Engström et al. 1964, Oliet & Sorin 1969). However, these studies were limited by the use of bacteriological methods that could not recover many of the bacteria normally present in the root canal. Apart from ensuring thorough aseptic control during endodontic procedures and bacteriological sampling (Möller 1966), the use of methods that recover all microorganisms is critical. When advanced methods for cultivating anaerobic bacteria have been used to study the root canal flora, obligate anaerobic bacteria have been shown to form a high proportion of the total flora in infected root canals (Kantz & Henry 1974, Wittgow & Sabiston 1975, Sundqvist 1976). Most of the bacteria isolated from infected root canals are oxygen sensitive and cannot be cultivated using conventional bacteriological methods Carlsson et al. 1977). In previous studies that have evaluated the influence of infection on the outcome of the treatment, bacteriological techniques were used that were unfavourable for the recovery of anaerobic bacteria (Zeldow & Ingle 1963, Engström et al. 1964, Oliet & Sorin 1969, Heling & Shapira 1978). Therefore, the presence of bacteria which might have been important for the outcome of treatment might have been missed and cases that seemingly contained no bacteria could in fact have harboured persisting microorganisms.
Another important factor in determining the outcome of endodontic therapy is the use of a long enough observation period after the treatment has been completed. The recovery of the periapical tissues to a healthy condition is a dynamic process and it is possible that a premature evaluation of periapical healing might include teeth in which the repair process has not yet stabilized. Many follow-up studies are limited by an observation period that is too short to reveal the true outcome of treatment. Because most lesions resolve within 4–5 years after therapy, it is considered desirable to have a follow-up period of at least 4 years (Strindberg 1956, Byström et al. 1987, Sjögren 1996).
Our study investigated the outcome of teeth treated by endodontic therapy, some of which contained cultivable bacteria at the time of root filling. Teeth with infected root canals and apical periodontitis were treated by instrumentation and antibacterial irrigation and were root-filled during the same appointment. Advanced anaerobic culturing techniques were used to check the canals for the presence of microorganisms initially and then just prior to obturation. The treated teeth were followed-up for 5 years. In some cases where treatment failed, a combination of microbiological techniques and microscopy was used to determine the possible reasons for failure.
Material and methods
Clinical material
Fifty-five single-rooted teeth were treated, all of which had intact pulp chamber walls, necrotic pulps and radiographic evidence of periapical bone lesions. Radiographic examination was carried out using the paralleling technique with Kodak Ultraspeed film (22 × 35 mm) in a film holder (Eggen 1974). The size of each lesion was calculated by taking the average of the lesions’ largest dimension and the extent in the direction perpendicular to the largest dimension.
Endodontic treatment
Rubber dam and an aseptic technique were used throughout the endodontic treatment. After gaining access to the root canal the working length was determined, taking care not to penetrate the apical foramen. An initial bacteriological sample was taken from the root canal. Narrow root canals were initially enlarged by hand filing until a size 20 file could be introduced to the working length. The coronal part of the root canal was flared with files energized by ultrasonics (Electro Medical Systems SA, Switzerland) as described previously (Sjögren & Sundqvist 1987) using 0.5% sodium hypochlorite as an irrigant. Hand files were used to shape the apical portion of the canal to a size 40 Hedström or larger at the working length. After mechanical instrumentation and irrigation of the canal, any residual sodium hypochlorite was inactivated by rinsing the canal with a 5% sodium thiosulphate solution (Möller 1966). A post-instrumentation sample was taken from the canal, after which the canal was filled with gutta-percha using the lateral condensation technique. The master cone was adapted to the canal by dipping it in rosin chloroform, and then multiple accessory cones were laterally condensed after having been softened in chloroform. When a post space was required, the root filling was removed to the desired length and a 1–2-mm-thick zinc-oxide-eugenol plug was applied over the root filling. Subsequent restorative treatment of the tooth was performed without rubber dam isolation and a nonaseptic technique.
Bacteriological sampling
Bacteriological samples were obtained from root canals in a procedure similar to that described previously (Byström & Sundqvist 1981, 1983). Briefly, sterile saline solution was introduced into the root canal by syringe and the fluid in the canal was absorbed with charcoaled paper points and transferred to a sampling tube. The initial sample, taken before canal preparation, was placed in a liquid thioglycolate medium (11260; Baltimore Biological Laboratories, Cockeysville, MD USA) supplemented with agar to prevent oxygen diffusion (Carlsson & Sundqvist 1980). This medium is highly effective in reducing oxygen, so that toxic intermediates of oxygen do not accumulate even if the medium is exposed to air for a short time (Carlsson et al. 1978).
Three post-instrumentation samples were taken from each canal. Two samples were transferred to peptone yeast extract glucose (PYG) broth (Holdeman et al. 1977), using precautions to avoid oxygen contamination (Fulghum 1971), and a third sample was transferred to the agar-supplemented thioglycolate medium. In one case a sample was taken from the periapical region at the time of operation. After raising a flap, the purulent contents of the lesion were aspirated with a syringe. The syringe was transferred to the laboratory where it was introduced into an anaerobic box, and the contents were treated in the same manner as the root canal samples.
Microbiological examination of the samples
All samples were introduced into an anaerobic box with an atmosphere of 10% hydrogen and 5% carbon dioxide in nitrogen. One PYG broth tube was agitated in a mechanical mixer until the paper points disintegrated, and 10-fold serial dilutions were made in buffered salt solution (Holdeman et al. 1977). Aliquots from the PYG broth and from each of the dilutions were inoculated on to blood agar plates (Holdeman et al. 1977). The plates were incubated for at least 10 days in the box at 37°C and were observed daily for growth. If no growth had occurred after 1 week, new blood agar plates were inoculated from the second PYG broth tube. A root canal sample was considered free of living bacteria when no growth was observed on the plates inoculated from both PYG broth tubes and the agar-supplemented thioglycolate medium.
When growth occurred, different colony morphotypes on the blood agar plates were isolated. The bacterial isolates were identified according to standard methods (Cowan 1974, Holdeman et al. 1977, Krieg & Holt 1984, Sneat et al. 1986, Hill et al. 1987). The identity of some strains was confirmed by comparing the mobility of their soluble proteins in sodium dodecyl sulphate-polyacrylamide gel electrophoresis (Laemmli 1970) with that of reference strains. The initial canal samples were not subcultivated, because these samples were taken only to confirm that the canals had been infected before treatment. All bacteria in post-instrumentation samples were identified to the species level, except for two cases where species level identification was not done because of technical errors.
Follow-up examination
Patients were recalled yearly for clinical and radio-graphic examination of the root-filled teeth. At the recall appointment, the type of restoration and any clinical signs or symptoms associated with the teeth were recorded. Radiographs were taken with the same X-ray unit using the long-cone technique and standardized exposure and processing to obtain optimal diagnostic quality of the radiographs. Strindberg’s (1956) criteria were used to judge the success rate of treatment. Briefly, treatment was considered successful when (i) the contours, width and structure of the periodontal margin were normal, or when (ii) the periodontal contours were widened mainly around an excess of filling material. All cases in which those criteria were not fulfilled were judged as unsuccessful. The cases were followed-up for 5 years if complete healing had not taken place earlier.
Radiographs were analysed separately by two independent observers using a light-box with variable illumination and a magnification viewer. The observers were calibrated as described by Halse & Molven (1986). In cases of disagreement, the two observers discussed the case in an effort to come to a consensus. If the observers still disagreed on a particular case, the opinion of a third specialist was taken as final. Statistical analysis of treatment outcomes was performed using a chi-squared test with Yates’ correction. Logistic regression analysis was also used to determine the influence of the apical level of the root filling and the bacteriological status on treatment outcomes.
Histology and immunohistochemistry
Biopsy specimens from three cases that showed no signs of periapical healing were analysed by histology. Two of these specimens, from teeth that were infected at the time of root filling (Table 2: EK11, EK12) were subjected to immunohistochemical analysis using methods described previously (Sjögren et al. 1988). Briefly, biopsy tissue was fixed in 4% buffered formalin, embedded in paraffin, cut into 5-µm-thick sections and stained with haematoxylin and eosin. Because bacteria of the species Actinomyces israelii had been isolated from the root canals at the time of root filling, the presence of the same bacteria was studied in the surgical tissue with anti-serum to A. israelii using the avidin-biotin-peroxidase method (VectastainTM ABC Kit; Vector Laboratories Inc., Burlingame, CA, USA). The antiserum was obtained from Centre for Disease Control (Atlanta, GA, USA). Controls were made as described earlier (Sjögren et al. 1988).
The third specimen was obtained from a case where no bacteria could be detected at the time of root filling (Fig. 3). This biopsy was from a lower second premolar in a 43-year-old male. The tooth had been nonvital and carious with an extensive radiolucency at the periapex before treatment. After endodontic therapy there was initially a slight reduction in size, but the lesion did not show any further radiographic signs of improvement after 3 years so the tooth was committed for apical surgery. The tooth had remained symptomless over the entire follow-up period. During surgery the lesion was unintentionally separated from the root tip and a puslike material oozed from the inflamed mass of soft tissue. Some pus was aspirated into a sterile syringe for microbiological analysis. Thereafter, the root tip and lesion were fixed by immersion in half-strength Karnovsky’s fixative and processed for electron microscopy.
Results
Bacteria were initially present in the root canals of all 55 treated teeth. At the completion of instrumentation, 22 root canals (40%) still contained recoverable bacteria and these were identified to species level in 20 of 22 root canals (Table 1). The number of species in root canals with persistent infection ranged from one to six, and 93% of these strains were anaerobic. In most cases there was a low number of bacterial cells in the sample, so that in eight cases bacteria were detected only after enrichment growth in the sampling media and in nine other cases the number of bacterial cells was 102–103. Only three samples had more than 104 bacterial cells (SN and EK12, Table 2; MW, Table 3).
Fifty-three teeth (96%) could be followed-up. Forty-four of the lesions healed completely and nine cases, all of which were symptomless, were judged to be failures. Seven of the failures were found amongst the 22 cases from which bacteria had been isolated at the time of root filling. Thus the success rate for teeth with positive samples at the time of root filling was 68%. The bacteria isolated from the root canals of the teeth where treatment failed are presented in Table 2, and one of the cases is shown in Fig. 1. Histological analysis of failure cases EK11 and EK12 (Table 2) revealed that filamentous microorganisms, which stained positively with the antiserum to A. israelii, were present in the periapical tissue. Bacteria, which had been isolated from the root canals of teeth which healed, in spite of yielding positive samples, are presented in Table 3. One of these cases is shown in Fig. 2.
Table 1 Bacteria recovered from 20 root canals at obturationa
| Species | No. | Species | No. |
| Enterococcus faecalis | 1 | Propionibacterium acnes | 2 |
| Streptococcus constellatus | 1 | Propionibacterium propionicum | 2 |
| Peptostreptococcus anaerobius | 3 | Bacteroides gracilis | 3 |
| Peptostreptococcus micros | 4 | Bacteroides sp.b | 2 |
| Eubacterium alactolyticum | 5 | Prevotella buccae | 2 |
| Eubacterium lentum | 3 | Prevotella oralis | 1 |
| Eubacterium nodatum | 2 | ||
| Eubacterium timidum | 1 | Fusobacterium nucleatum | 5 |
| Actinomyces israelii | 2 | Campylobacter rectus | 4 |
| Actinomyces naeslundii | 1 | ||
| Actinomyces odontolyticus | 1 |
aBacteria persisting in two root canals not identified. bAsaccharolytic strains with few characteristics, not identified to species level.
Table 2 Bacteria present at the time of root filling in cases that failed to heal
| Case | Persisting strains | |
| SN | Eubacterium alactolyticum | |
| Eubacterium lentum | ||
| Eubacterium timidum | ||
| EK 11 | Actinomyces israelii | |
| Prevotella buccae | ||
| EK 12 | Actinomyces israelii | |
| Actinomyces odontolyticus | ||
| Eubacterium nodatum | ||
| MAY | Eubacterium alactolyticum | |
| Peptostreptococcus anaerobius | ||
| Peptostreptococcus micros | ||
| Bacteroides gracilis | ||
| AN | Fusobacterium nucleatum | |
| Prevotella oralis | ||
| BA | Eubacterium lentum | |
| Campylobacter rectus | ||
| BOF | NDa |
aNo identification done.
Twenty-nine of 31 teeth with negative samples at the time of root filling healed; a success rate of 94%. One of the two failure cases from this group is shown in Fig. 3. This case had shown no signs of periapical healing after a period of 3 years and was therefore scheduled for surgery. A bacteriological sample taken from the periapical region at operation showed growth of Actinomyces odontolyticus, Streptococcus constellatus, Propionibacterium acnes and a Campylobacter species. Direct microscopy of the sample revealed bacterial aggregates with intermeshed filaments. Histological analysis of the root tip revealed that the root canal was filled with gutta-percha and that one of the lateral canals at the root tip was clogged with bacteria.
Table 3 Bacteria present at the time of root filling in that healed successfully cases
| Case | Persisting strains | Case | Persisting strains | |||
| EAN | NDa | SF | Actinomyces naeslundii | |||
| Peptostreptococcus micros | ||||||
| Campylobacter rectus | ||||||
| MW | Eubacterium nodatum | OJ | Eubacterium alactolyticum | |||
| Bacteroides sp. | ||||||
| PS | Peptostreptococcus anaerobius | LJ | Fusobacterium nucleatum | |||
| Eubacterium alactolyticum | ||||||
| Fusobacterium nucleatum | ||||||
| Prevotella buccae | ||||||
| Campylobacter rectus | ||||||
| Bacteroides sp. | GJ | Propionibacterium propionicum | ||||
| LA | Eubacterium alactolyticum | GS | Propionibacterium acnes | |||
| Streptococcus constellatus | ||||||
| RH | Peptostreptococcus anaerobius | TR | Bacteroides gracilis | |||
| Peptostreptococcus micros | ||||||
| AA | Propionibacterium acnes | IN | Enterococcus faecalis | |||
| Eubacterium lentum | Peptostreptococcus micros | |||||
| Fusobacterium nucleatum | ||||||
| PB | Propionibacterium propionicum | AMJ | Fusobacterium nucleatum | |||
| Bacteroides gracilis | ||||||
| Campylobacter rectus |
aNo identification done.
Assessment of the apical level of the root filling revealed that the majority of teeth were obturated within 2 mm of the apex. In 10 teeth there was a slight overfilling, but in each of these cases the extension of excess material into the periapical tissue was less than 1 mm. Five of the overfilled teeth had positive and five had negative samples at the time of obturation. The slight overfilling appeared to have no influence on the outcome, because all 10 teeth root-filled with excesses were successful. Of the remaining 43 cases, there was no statistical difference in outcome between the teeth root-filled ‘flush’ or within 2 mm from the apex. Statistical analysis revealed that the absence of bacteria at the time of root filling increased the probability of a successful outcome (P = 0.041).
The size of the initial lesion in relation to cases that healed or failed is shown in Table 4. The preoperative size of the periapical lesion apparently had no influence on the outcome of treatment.
Table 4 Preoperative size of periapical lesions in relation to the outcome of treatment
| Bacteriological sample at root filling | Initial size of periapical lesion (mm) | |||||
| Healed lesions | No healing | |||||
| Mean | Range | Mean | Range | |||
| Positive | 6.5 | 2.5–11 | 5.7 | 3–7.5 | ||
| Negative | 4.6 | 2–10 | 3.5 & 8.5a | |||
aTwo cases only, actual sizes.
Discussion
Although the presence of bacteria is known to be essential for the development of apical periodontitis, the influence of persisting infection at the time of root filling on the outcome of endodontic treatment has not been thoroughly evaluated in well-controlled studies. It is generally accepted that the success of endodontic treatment relies on thorough elimination of bacteria from the root canal system before root filling, largely because of the role bacteria play in the pathogenesis of apical periodontitis. The present study combines the use of advanced anaerobic bacteriological techniques on optimally treated cases with a long follow-up period and a high recall rate to evaluate the role of persistent microorganisms on the outcome of treatment. Importantly, this work has revealed that the bacteriological status of the root canal at the time of root filling is a critical factor in determining the outcome of endodontic treatment.
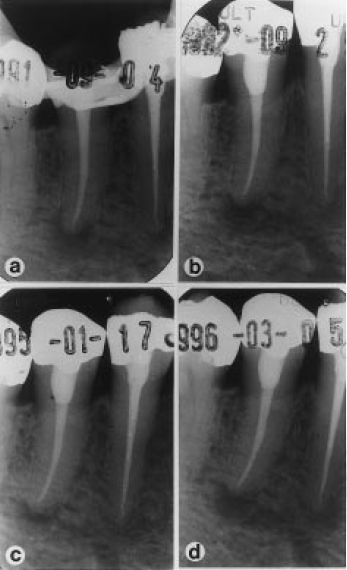
Fig. 1 Follow-up radiographs of a failed treatment on a lower second premolar tooth (case AN, Table 2). A sample taken from the root canal at the time of obturation (a) yielded a positive culture containing the species F. nucleatum and P. oralis. At subsequent reviews over 12, 41, and 54 months, no healing of the periapical lesion was observed on check radiographs (b, c, d).
The success rate of teeth filled with a negative culture before obturation of the root canal was 94%, which was statistically significantly higher than the success rate of 68% for teeth with a positive culture at the time of root filling (P = 0.023). This concords with the findings of Heling & Shapira (1978), who noticed that infection at the time of root filling had a negative influence on treatment outcome. Their data also showed that an observation period of 4–5 years is required for the full impact of the persisting bacteria on the prognosis to be revealed.
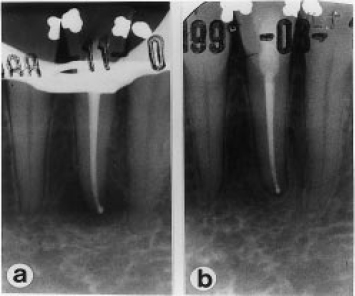
Fig. 2 Follow-up radiographs of a successful treatment on a lower left second incisor tooth (case RH, Table 3). A sample taken from the root canal at the time of obturation (a) yielded a positive culture containing the species P. anaerobius and P. micros. A check radiograph taken 33 months later (b) shows periapical healing.
In our study, the interpretation of recall radiographs was unequivocal because the long follow-up period afforded a clear interpretation of whether or not there was complete periapical healing. It was apparent that the preoperative size of the periapical lesion had no influence on the outcome of treatment, confirming previous reports that have had an extended follow-up period (Strindberg 1956, Sjögren et al. 1990). We found a greater impact of infection on prognosis than that described previously, probably because of the combination of an extended observation period and the use of advanced bacteriological techniques, which favour the recovery of bacteria that might have been missed with culturing techniques used in earlier studies (Zeldow & Ingle 1963, Engström et al. 1964, Bender et al. 1964, Oliet & Sorin 1969).
Many of the factors previously identified as being responsible for an impaired healing rate (Sjögren et al. 1990) were surmounted in the cases described in our study. One of these factors was the apical level of the root filling. In an earlier study (Sjögren et al. 1990), teeth with apical periodontitis that were underfilled by more than 2 mm from the apex had a significantly lower success rate than teeth filled within 0–2 mm of the apex. However, in our study none of the treated teeth could be classified as underfilled because 43 of 53 recalled teeth were obturated within 2 mm of the apex. Overfilling has also been identified as a factor associated with a lower success rate (Sjögren et al. 1990); however, in the present study all 10 teeth classified as overfilled healed completely. The most likely reason for the success of these cases, despite overfilling, is the minor volume of excess root filling material extruded into the periapical tissue, which in all cases was very slight. The risk of a foreign body reaction of any significance was therefore low. Furthermore, any correlative factors that may accompany or lead to overfilling, such as apical over-instrumentation or extrusion of canal debris into the periapical tissues, were minimized, because canals were instrumented to their full length and were treated to a high standard by an experienced specialist. The level of root filling seen in Fig. 2 is representative of the slight overfilling observed in many of these cases. It is possible that the number of teeth in the overfilled group was too small for any influence to be detected; however, even if the overfilled teeth had a negative impact and were therefore excluded from statistical calculations of success, analysis of the remaining 43 cases disclosed that the absence of bacteria at the time of root filling increased the likelihood of a successful outcome (P = 0.041).
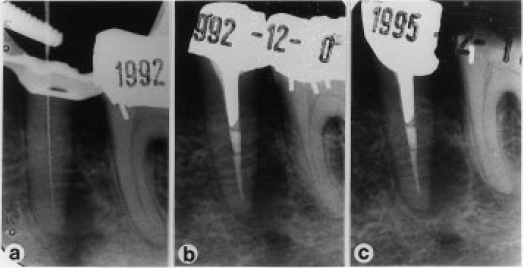
Fig. 3 Follow-up radiographs of a failed treatment on a lower second premolar tooth. No bacteria were isolated in a post instrumentation sample (a, b). At subsequent reviews over 34 months no healing of the periapical lesion was observed (c).
Of other factors associated with failure (Sjögren et al. 1990), few were applicable to the cases reported here. Canal obliteration and root resorption were two factors demonstrated to have an influence on prognosis for teeth with apical periodontitis (Sjögren et al. 1990); however, the infected and noninfected cases in our study were comparable in this respect. Another factor previously correlated with failure is the way the tooth is restored (Sjögren et al. 1990). In our study, teeth that required a post retained restoration were additionally sealed before restoration with a zinc-oxide-eugenol plug coronal to the root filling to avoid potential bacterial leakage. Thus, all possible factors that may have affected the prognosis were well controlled, apart from the critical variable under investigation in this study - infection at the time of root filling.
Given that the cases in the present study were carefully controlled and were distinguished essentially by the presence or absence of infection at the time of root filling, it seems reasonable to deduce that the presence of infection was the reason for the difference in success rates between the treated teeth. However, recent studies have highlighted the possibility that other factors apart from intraradicular infection, such as extraradicular infection, foreign body reactions and true cysts can contribute to failure (Sjögren et al. 1988, Nair et al. 1990b, Nair et al. 1993). For this reason, where clinically feasible, teeth that failed to heal were treated by surgery and the material recovered was subjected to microbiological and histological analysis. Material was available in three cases: two from the group of seven failures with infection and one of the two failures without infection at the time of root filling. Of the cases from the group with infection at the time of root filling, two teeth that had harboured A. israelii in their root canals at root filling were shown by histology to have developed periapical actinomycosis. It is well known that bacteria of this species have the ability to establish extraradicular infections in the periapical tissue and thereby prevent healing after conventional endodontic treatment (Sundqvist & Reuterwing 1980, Happonen 1986, O’Grady & Reade 1988). With the exception of A. israelii, there was no evidence suggesting that other bacteria were specifically implicated in failure cases.
Although nonmicrobial reasons might have been suspected for failure in teeth with no recoverable bacteria at the time of obturation, when surgery was performed on one of these cases a periapical abscess was present (Fig. 3). The bacterium A. odontolyticus dominated the bacterial flora of the abscess. There is a risk that the sample might have been contaminated because it was obtained after the flap had been raised (Möller 1966). But the fact that an abscess was detected at operation, that Actinomyces-like colonies were observed by direct microscopy of the sample, and that electron microscopy revealed bacteria filling an apical lateral canal, strongly point to the probability that the reason for the lack of healing in this case was a persisting infection. In a previous study, microorganisms were shown to prevent postoperative healing when they were hidden in apical branches of the root canal or in voids adjacent to the root filling (Nair et al. 1990a). The bacteria responsible for the failure of this case were also located in an apical lateral canal. The presence of bacteria in this location might explain the apparent failure of the sampling procedure to detect these bacteria. An explanation for the second failure case could not be determined because the patient did not want to have the tooth operated on or conventionally retreated.
Fifteen of 22 teeth that contained bacteria at the time of root filling eventually healed. This raises the question about the fate of the bacteria in these root canals. Many bacteria are destined to die, either from exposure to or by being entombed within the obturation material. If the bacteria perish in one of these ways, a favourable outcome can be expected for the case. Rosin, which is present in rosin-chloroform, has an antibacterial effect (Johansson et al. 1995), and the bacteria might not have survived the application of rosin-chloroform. Furthermore zinc and zinc compounds present in gutta-percha also possess antibacterial activity (Moorer & Genet 1982), an effect which is increased by resin acids and oleoresins (Johansson et al. 1995). Another possibility is that the bacteria were entombed or could not access suitable nutritional substrate after canal obturation and then died. If the bacteria survived, it is also possible that they could not reach the periapical tissues to cause clinical infection because of difficult access, low pathogenicity or low numbers.
The number and diversity of bacterial cells remaining in the root canal is important because a low number and limited variety of bacteria, with which to maintain co-operative nutritional relationships, would be unfavourable for their survival. Previous studies have shown that the number of bacterial cells persisting after instrumentation and irrigation with sodium hypochlorite is usually low in teeth with chronic periapical access (Byström & Sundqvist 1983, 1985, Sjögren & Sundqvist 1987). In the present study, the number of surviving bacteria was usually low. An estimation of the number of surviving bacteria could only be made when growth occurred on the solid media inoculated from serially diluted samples, but in cases where bacteria could be detected only after enrichment growth it must be assumed that the number of bacteria was very low. However, in all cases where viable bacteria remain in the root canal, there is always that they may maintain a periapical inflammation.
Although this study has clearly shown that a negative bacteriological sample at the time of root filling is important for success, it does not mean that routine culturing is required in normal clinical treatment. This is because earlier studies have shown that infection can be reliably eliminated from the root canal by using aseptic working techniques, antimicrobial irrigation during instrumentation and an antimicrobial dressing between visits (Byström & Sundqvist 1983, 1985, Byström et al. 1985, Sjögren et al. 1991). Sixty per cent of the instrumented canals in this study were bacteria-free by the end of the appointment, which corroborates earlier work on the effectiveness of instrumentation with sodium hypochlorite (Byström & Sundqvist 1985, Sjögren & Sundqvist 1987). There was no indication in our study, or in previous ones (Byström & Sundqvist 1981, 1983, 1985, Sjögren & Sundqvist 1987, Sjögren et al. 1991), that specific bacteria were resistant to the biomechanical treatment. The most common species in post-instrumentation samples were Fusobacterium nucleatum, Peptostreptococcus micros and Eubacterium alactolyticum, but these species also belong to the most prevalent ones recovered from infected root canals (Sundqvist 1992, Wasfy et al. 1992, Sato et al. 1993). Whilst instrumentation and antimicrobial irrigation will significantly reduce the microflora within the root canal, such procedures alone are insufficient to eradicate bacteria from the root canal. The most efficient way to accomplish thorough elimination of bacteria is to use an antibacterial dressing between visits, as demonstrated earlier (Byström & Sundqvist 1985, Sjögren et al. 1991).
It has been argued that there is insufficient evidence to support the clinical application of special measures to eradicate all bacteria in the canal (Peters et al. 1995). However, the present study has shown that the success rate of endodontic treatment is 26% higher if the root canal is free from bacteria, than if it is infected, at the time of root canal obturation. Not every tooth that contains bacteria at the time of root filling will result in failure; however, these findings show that in some cases bacteria will survive in sufficient numbers to cause clinically significant pathology. Because the presence or absence of infection cannot normally be determined at the time of canal obturation, it is important that canal preparation be directed towards the complete elimination of all bacteria from the root canal system so that the best possible prognosis can be assured in endodontic treatment. The results also affirm the findings of previous studies which have shown that the most probable reason for failure of endodontic treatment is the presence of a persisting infection (Nair et al. 1990a, Sjögren 1996). Despite providing optimal endodontic therapy for teeth in this study it was not possible to eradicate reliably all infection from the root canal in one treatment visit, which suggests that filling of initially infected root canals should be delayed until after a suitable period of medication with an antimicrobial dressing.
Acknowledgements
We thank Dr R.-P. Happonen, Turku for performing the immunohistochemical analysis, Dr P. N. R. Nair, Zürich for performing the histopathological analysis, and Ms Lilian Palmqvist and Ms Sonia Andersson for excellent clinical assistance.
References
BENDER IB, SELTZER S, TURKENKOPF S (1964) To culture or not to culture? Oral Surgery, Oral Medicine, Oral Pathology 18, 527–40.
BYSTRÖM A, SUNDQVIST G (1981) Bacteriological evaluation of the efficacy of mechanical root canal instrumentation in endodontic therapy. Scandinavian Journal of Dental Research 89, 321–8.
BYSTRÖM A, SUNDQVIST (1983) Bacteriologic evaluation of the effect of 0.5 per cent sodium hypochlorite in endodontic therapy. Oral Surgery, Oral Medicine and Oral Pathology 55, 307–12.
BYSTRÖM A, SUNDQVIST (1985) The antibacterial action of sodium hypochlorite and EDTA in 60 cases of endodontic therapy. International Endodontic Journal 18, 35–40.
BYSTRÖM A, CLAESSON R, SUNDQVIST G (1985) The antibacterial effect of camphorated paramonochlorophenol, camphorated phenol and calcium hydroxide in the treatment of infected root canals. Endodontics and Dental Traumatology 1, 170–75.
BYSTRÖM A, HAPPONEN R-P, SJÖGREN U, SUNDQVIST G (1987) Healing of periapical lesions of pulpless teeth after endodontic treatment with controlled asepsis. Endodontics and Dental Traumatology 3, 58–63.
CARLSSON J, SUNDQVIST G (1980) Evaluation of methods of transport and cultivation of bacterial specimens from infected root canals. Oral Surgery, Oral Medicine and Oral Pathology 49, 451–4.
CARLSSON J, FRÖLANDER F, SUNDQVIST G (1977) Oxygen tolerance of anaerobic bacteria isolated from necrotic dental pulps. Acta Odontologica Scandinavia 35, 139–45.
CARLSSON J, NYBERG G, WRETHÉN J (1978) Hydrogen peroxide and superoxide radical formation in anaerobic broth media exposed to atmospheric oxygen. Applied and Environmental Microbiology 36, 223–9.
COWAN ST (1974) Cowan and Steel’s Manual for the Identification of Medical Bacteria, 2nd edn. Cambridge: Cambridge University Press.
EGGEN S (1974) Röntgenografiske tannmålinger i daglig praksis ved hjälp av standardisert parallell-teknikk og en kalibrert målelinjal. Tandläkartidningen 66, 10–12.
ENGSTRÖM B, HÅRD AF, SEGERSTAD L, RAMSTRÖM G, FROSTELL G (1964) Correlation of positive cultures with the prognosis for root canal treatment. Odontologisk Rev 15, 257–70.
FULGHUM RS (1971) Mobile anaerobe laboratory. Applied Microbiology 21, 769–70.
HALSE A, MOLVEN O (1986) A strategy for the diagnosis of periapical pathosis. Journal of Endodontics 12, 534–8.
HAPPONEN, R-P (1986) Periapical actinomycosis: a follow-up study of 16 surgically treated cases. Endodontics and Dental Traumatology 2, 205–9.
HELING B, SHAPIRA J (1978) Roentgenologic and clinical evaluation of endodontically treated teeth with or without negative culture. Quintessence International 11, 79–84.
HILL GH, AYERS OA, KOHAN AP (1987) Characteristics and sites of infection of Eubacterium nodatum, Eubacterium timidum, Eubacterium brachy, and other asaccharolytic eubacteria. Journal of Clinical Microbiology 25, 1540–45.
HOLDEMAN LV, CATO EP, MOORE WEC (1977) Anaerobe Laboratory Manual, 4th edn. Blacksburg: Virginia Polytechnic Institute and State University.
JOHANSSON A, SUNZEL B, HOLM SE, SÖDERBERG T, GREF R (1995) Antimicrobial screening of zinc in the absence or presence of oleoresins and various resin acids. APMIS 103, 1–9.
KAKEHASHI S, STANLEY HR, FITZGERALD RJ (1965) The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surgery, Oral Medicine and Oral Pathology 20, 340–49.
KANTZ WE, HENRY CA (1974) Isolation and classification of anaerobic bacteria from intact pulp chambers of non-vital teeth in man. Archives of Oral Biology 19, 91–6.
KRIEG NR, HOLT JG, eds (1984) Bergey’s Manual of Systematic Bacteriology, vol. 1. Baltimore: Williams & Wilkins.
LAEMMLI UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–85.
MÖLLER ÅJR (1966) Microbiological examination of root canals and periapical tissues of human teeth. Odontologisk Tidskrift 74, (Suppl.), 1–380.
MÖLLER ÅJR, FABRICIUS L, DAHLÉN G, ÖHMAN AE, HEYDEN G (1981) Influence on periapical tissues of indigenous oral bacteria and necrotic pulp tissue in monkeys. Scandinavian Journal of Dental Research 89, 475–84.
MOORER WR, GENET JM (1982) Antibacterial activity of gutta-percha cones attributed to the zinc oxide component. Oral Surgery, Oral Medicine and Oral Pathology 53, 508–17.
NAIR PNR, SJÖGREN U, KREY G, KAHNBERG K-E, SUNDQVIST G (1990a) Intraradicular bacteria and fungi in root-filled asymptomatic human teeth with therapy-resistant periapical lesions: a long-term light and electron microscopic follow-up study. Journal of Endodontics 16, 580–88.
NAIR PNR, SJÖGREN U, KREY G, SUNDQVIST G(1990b) Therapy-resistant foreign body giant cell granuloma at the periapex of a root-filled human tooth. Journal of Endodontics 16, 589–95.
NAIR PNR, SJÖGREN U, SCHUMACHER E, SUNDQVIST G (1993) Radicular cyst affecting a root-filled human tooth: a long-term post-treatment follow-up. International Endodontic Journal 26, 225–33.
O’GRADY JF, READE PC (1988) Periapical actinomycosis involving Actinomyces israelii. Journal of Endodontics 14, 147–9.
OLIET S, SORIN SM (1969) Evaluation of clinical results based upon culturing root canals. Journal of British Endodontic Society 3, 3–6.
PETERS LB, WESSELINK PR, MOORER WR (1995) The fate and the role of bacteria left in root dentinal tubules. International Endodontic Journal 28, 95–9.
SATO T, HOSHINO E, UEMATSU H, NODA T (1993) Predominant obligate anaerobes in necrotic pulps of human deciduous teeth. Microbial Ecology in Health and Disease 6, 269–75.
SJÖGREN U (1996) Success and failure in endodontics. (Odontological Dissertation No. 60.) Umeå: Umeå University, Sweden.
SJÖGREN U, SUNDQVIST G (1987) Bacteriologic evaluation of ultrasonic root canal instrumentation. Oral Surgery, Oral Medicine and Oral Pathology 63, 366–70.
SJÖGREN U, HAPPONEN RP, KAHNBERG KE, SUNDQVIST G (1988) Survival of Arachnia propionica in periapical tissue. International Endodontic Journal 21, 277–82.
SJÖGREN U, HÄGGLUND B, SUNDQVIST G, WING K (1990) Factors affecting the long-term results of endodontic treatment. Journal of Endodontics 16, 498–504.
SJÖGREN U, FIGDOR D, SPåNGBERG L, SUNDQVIST G (1991) The antimicrobial effect of calcium hydroxide as a short-term intracanal dressing. International Endodontic Journal 24, 119–25.
SNEAT PHA, MAIR NS, SHARPE ME, HOLT JG, eds (1986) Bergey’s Manual of Systematic Bacteriology, vol. 2. Baltimore: Williams & Wilkins.
STRINDBERG LZ (1956) The dependence of the results of pulp therapy on certain factors. An analytic study based on radiographic and clinical follow-up examinations. Acta Odontologica Scandinavia 14 (Suppl. 21), 1–175.
SUNDQVIST G (1976) Bacteriological studies of necrotic dental pulps. (Odontological Dissertation No. 7.) Umeå: University of Umeå, Sweden.
SUNDQVIST G (1992) Associations between microbial species in dental root canal infections. Oral Microbiology and Immunology 7, 257–62.
SUNDQVIST G, REUTERWING C-O (1980) Isolation of Actinomyces israelii from periapical lesion. Journal of Endodontics 6, 602–6.
WASFY MO, MCMAHON KT, MINAH GE, FALKLER WA (1992) Microbiological evaluation of periapical infections in Egypt. Oral Microbiology and Immunology 7, 100–105.
WITTGOW WC, SABISTON CB (1975) Microorganisms from pulpal chambers of intact teeth with necrotic pulps. Journal of Endodontics 1, 168–71.
ZELDOW BJ, INGLE JI (1963) Correlation of the positive cultures to the prognosis of endodontically treated teeth: a clinical study. Journal of the American Dental Association 66, 9–13.
____________
Correspondence: Professor G. Sundqvist, Department of Endodontics, Faculty of Odontology, Umeå University, S-901 87 Umeå, Sweden (e-mail: [email protected] ).