Starvation survival and recovery in serum of Candida albicans compared with Enterococcus faecalis
Donna Richards, BDSc, GradDipClinDent, DCD,a John K. Davies, BSc, PhD,b and David Figdor, MDSc, FRACDS, DipEndo, PhD, FASM,a,b Melbourne, Australia
UNIVERSITY OF MELBOURNE AND MONASH UNIVERSITY
Objective. Candida albicans has been a common isolate in posttreatment disease, usually as a monoinfection of the root filled canal. A factor likely to contribute to its pathogenic potential in posttreatment infection is an ability to endure starvation and use serum as a nutritional source. This study evaluated the starvation-survival behavior, growth, and recovery in human serum of C. albicans and compared it with Enterococcus faecalis.
Study design.Varying cell densities of C. albicans and E. faecalis were suspended in 5% human serum or water for 4-6 months. Starvation recovery was assessed by addition of 50% serum to starved cells. Cell survival was monitored by periodic removal of aliquots and viable counts.
Results.Initial cell density was important for starvation survival. Candida albicans and E. faecalis survived starvation in water for 6 months whenl density was the starting cel >105 and ≥108 colony-forming units (cfu)/mL, respectively.Both species thrived in 5% serum from low initial densities (>102 and >104 cfu/mL for C albicans and E. faecalis, respectively), and starvation-state cells recovered on addition of 50% serum.
Conclusion. Candida albicans is well suited for survival in nutrient-limited conditions and can use serum as a source of nutrition and for recovery from starvation. These findings parallel the behavior of E. faecalis, which possesses a similar capacity for starvation survival and growth in serum, traits that are of likely importance for their participation in posttreatment infection. (Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010;110:125-130)
The nutrient-rich milieu of the untreated infected pulp space typically supports a diverse polymicrobial mix dominated by anaerobes,1,2 in contrast to posttreatment infection, where the nutrient-limited environment within the obturated root canal usually contains a limited assortment of predominantly gram-positive facultative anaerobes of mostly single species.3,4 Enterococcus faecalis has been repeatedly implicated as the predominant species in monoinfection of teeth with persistent disease, but a relatively high prevalence of streptococci and yeast species have also been recovered from root-filled teeth with persistent apical periodontitis.3-7
The reduced assortment of species in teeth with persistent apical periodontitis3-8 points to a strong selection pressure favoring those species suited to survival in the nutrient-limited obturated root canal. A starvation-survival strategy would allow microorganisms to persist in the root-filled canal and to later, with access to nutrients, recover from the starved state.
Characterization of the starvation-survival response of E. faecalis has shown its ability to withstand starvation in water- or nutrient-limited media and that starvation-state cells could recover upon the addition of human serum.9 The starvation-survival capacity of other species implicated in persistent apical periodontitis is essentially unexplored, yet further investigation should provide a better understanding of the role of specific pathogenic properties necessary for involvement in posttreatment disease.
Candida albicans is a common inhabitant of the oral cavity (31%-44% of healthy subjects).10,11 Yet in the untreated infected root canal, it is an infrequent participant (2%-7%), as reported by both culture-based11-13 and polymerase chain reaction (PCR)-based14,15 studies, with 1 exception16 (21%). In posttreatment infections, a higher prevalence of 3%-18% with C. albicans has been described using culture methods3-7,11,17,18 and of 6%-9% by PCR.19,20 Potential pathogenic properties that favor a higher prevalence of fungi in posttreatment infection have been described in several reviews and include resistance to antimicrobial treatment and dentine adhesion and invasion.21-24
We hypothesized that other species found in monoinfections of root canals with persistent disease might also possess a starvation survival ability similar to E. faecalis. Therefore, this study sought to characterize the starvation-survival response of C. albicans and its ability to recover from starvation in the presence of human serum. These observations were compared to the starvation-survival and serum-recovery kinetics of E. faecalis.
MATERIALS AND METHODS
Microorganisms and culture conditions
Candida albicans (strain MFC14.2, Microbiology Department, Monash University) and E. faecalis JH2-2, derived from the parental strain JH2,25 were grown in brain-heart infusion (BHI) broth, incubated with shaking, or on BHI agar plates (Oxoid, Basingstoke, U.K.) in an aerobic environment at 37°C. All experiments were performed in triplicate, except long-term starvation of C. albicans with a starting cell density of 104 colony-forming units (cfu)/mL, which was performed in duplicate.
Growth and starvation of cells
For starvation assays, cells were harvested by centrifugation (3,200g, 10 min), washed twice in phosphate-buffered saline solution (PBS), and resuspended in sterile distilled water to a final suspension of 103-109 cfu/mL. Cell density was confirmed by viable counts. Starvation-survival kinetics were followed for 6 months.
Growth, survival, and recovery in human serum
Sera from 3 healthy human adults were pooled, inactivated at 56°C for 30 minutes, and stored at –80°C until used. Growth in pooled human serum (PHS) was determined by inoculation of mid-log-phase cells into 5% PHS (diluted in PBS). Long-term survival was determined for cell densities of 103-106 cfu/mL in 5% PHS, with stationary incubation at 37°C for 4 months.
Recovery of 7- and 14-day starved cells was assessed by serum supplementation (50% concentration after addition) to starvation cultures followed by incubation at 37°C. At preset intervals, aliquots were removed and survival determined by viable counts of serial dilutions in PBS and plating on BHI agar.
RESULTS
Starvation-survival kinetics
The kinetics of starvation survival for the lowest starting cell density of C. albicans and E. faecalis that survived in water for 6 months are shown in Fig. 1. Candida albicans survived 6 months’ starvation in water if the initial cell density was >105 cfu/mL. With starting densities of 106-109 cfu/mL, there was a gradual decline in cell numbers, but at 6 months there was still a viable cell population of ~105 cfu/mL (data not shown). Candida albicans did not survive starvation beyond 3 weeks if the starting density was = 104 cfu/mL (data not shown).
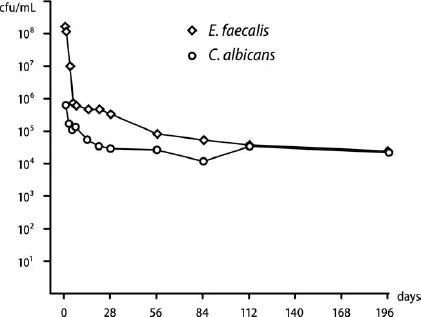
Fig. 1. Starvation survival of Candida albicans (6.3 × 105 cfu/mL; circles) and Enterococcus faecalis (2.2 × 108 cfu/mL; diamonds) in water for 6 months.
Enterococcus faecalis survived starvation in water for 6 months if the initial cell density was = 108 cfu/mL at the onset of starvation. At lower densities (106 and 107 cfu/mL) cell survival was short-lived and no cells were recovered at 5 and at 56 days, respectively (data not shown).
Growth in serum
Candida albicans thrived in 5% serum, even at low (102-104 cfu/mL) starting cell densities (Fig. 2, A). Cell numbers grew rapidly and stabilized at a steady population of about 105 cfu/mL over the 4-month observation period.
Inclusion of 5% serum sustained E. faecalis from low initial cell densities (104-106 cfu/mL) with a mild decline in the cell population to about 104 cfu/mL over 4 months (Fig. 2, B). At higher initial starting densities (3.5 × 107 and 2.8 × 108 cfu/mL), cell numbers stabilized at a higher level (~106 cfu/mL) for 1-4 months (data not shown).
Revival of starved cells by serum
The capacity of serum to revive starved cells was tested on cultures of low starting cell density that had been previously shown to be unable to survive starvation. The addition of PHS to 14-day starved C. albicans led to resurgent growth in the cell population (Fig. 3, A). With E. faecalis, introduction of serum to 7-day starved cells resulted in recovery and resumed growth (Fig. 3, B). This was in contrast to control addition of PBS, which had no effect on survival.
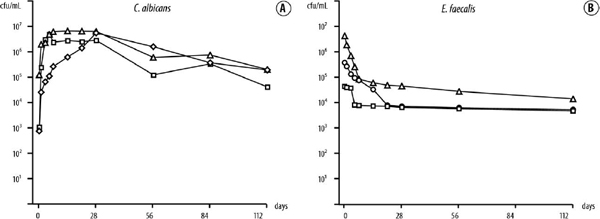
Fig. 2. A, Growth of Candida albicans in 5% serum for >4 months from initial cell densities of 7.8 × 102 (diamonds), 1.0 × 103 (squares), and 1.7 × 105 (triangles) cfu/mL. B, Growth of Enterococcus faecalis in 5% serum for >4 months from initial cell densities of 4.6 × 104 (squares), 3.8 × 105 (circles), and 3.4 × 106 (triangles) cfu/mL.
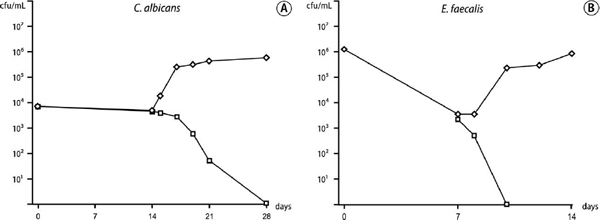
Fig. 3. A, Recovery of 14-day starvation-state cells of Candida albicans after addition at 14 days of serum (diamonds) and phosphate-buffered saline solution (PBS; control; squares); starved cell densities were 3.9 and 4.1 × 103 cfu/mL, respectively. B, Recovery of 7-day starvation-state cells of Enterococcus faecalis after addition at 7 days of serum (diamonds) and PBS control (squares); starved cell densities were 3.6 and 2.5 × 103 cfu/mL, respectively.
DISCUSSION
When selected microorganisms are recovered in greater prevalence from failed endodontic treatment cases than in untreated infected teeth, it implicates those species in the pathogenesis and maintenance of persistent apical periodontitis. The species proliferating in the root-filled canal presumably share properties that position them favorably for survival in this inhospitable ecologic niche. Previously, we surmised that an ability to endure long periods of starvation and use serum-like transudate for growth are factors favorable for pathogenesis, and we were able to demonstrate this capacity for E. faecalis.9 That E. faecalis survives starvation has been documented in other studies,26,27 yet limited information is available on starvation survival of C. albicans. Therefore, we examined the behavior of C. albicans under starvation conditions, and the results reveal that C. albicans has a corresponding capacity to endure starvation and exploit serum for growth and recovery from starvation.
Survival of cultivable cells in water for 6 months illustrated the starvation resilience of C. albicans (Fig. 1). This finding correlates well with an observation, described >70 years ago,28 that distilled water can be used as a storage method for yeasts and further reports showing long-term survival of C. albicans in distilled water for 3-10 years.29-31 Cell density at starvation onset was a significant factor for survival, where >105 cfu/mL was favorable for survival but at lower cell densities (=104 cfu/mL) C. albicans could not survive starvation beyond 3 weeks. The capacity of C. albicans for starvation survival compares favorably with E. faecalis, which can endure many months of starvation.9,27 Starvation survival for E. faecalis is also highly contingent on cell density at starvation onset.9,32 Interestingly, C. albicans survived starvation from a significantly lower initial cell density than E. faecalis (105 compared with 108 cfu/mL, respectively), which suggests a superior capacity for sustaining itself in limited numbers under nutrient-limited conditions.
Although there is no information regarding potential nutrition sources available for microorganisms embedded in the obturated canal, it is not unreasonable to think that tissue fluid derived from the periapical tissues may enter the root canal space to provide suitable substrate. Indeed, ultrastructural investigations of microbes involved in failed treatment cases have demonstrated that they are predominantly located in the apical region of the root canal space in voids or accessory canals.33,34 The proximity of microorganisms to the periapical granuloma is consistent with the idea that these microorganisms may be activated by adjacent host molecules35 and derive their nutrition from periapical tissue fluid. Therefore, we evaluated the potential of C. albicans and E. faecalis to use serum for survival and for recovery of starved cells.
Human serum sustained growth of both species for more than 6 months. Just 5% serum was enough to support growth of low numbers (7.8 × 102 cfu/mL) of C. albicans. Similarly, the results for growth of E. faecalis in human serum showed that 5% serum sustained the cells from low starting cell densities (4.6 × 104 cfu/mL) for >4 months, which correlated well with the findings of a previous study.9 Thus, the availability of even a low concentration of serum has the potential to dramatically prolong survival of low cell numbers of both species, compared with the requirement for a significantly higher starting density to endure absolute starvation.
In starvation-recovery experiments, starved C. albicans cells were rapidly revived by addition of 50% serum. Similarly, starved E. faecalis recovered with the nutritional support of serum, as shown previously.9 These results illustrate that the gradual demise of a small population of starved cells can be effectively reversed if they have the fortune to encounter a nutritional upshift from serum.
Environmental conditions, e.g., interaction with calcium and collagen components in dentin,36 have the potential to influence fungal behavior and growth form. Candida albicans grows in different forms, such as germ tubes, yeasts (blastospores), pseudo- and true hyphae, and chlamydospores, and, depending on the environmental cues, switching may occur among these morphotypes (except chlamydospores).36 Starvation and revival of starved C. albicans cells may induce morphologic switching, including to a hyphal growth form where cells remain attached to each other after division.24,37,38 Because cell survival was determined by enumeration of colony-forming units on non-Candida-specific plates, there was a possibility of an underestimation of cell numbers if starved C. albicans were not discrete single cells but had switched to a chained filamentous form.
Cell survival was assessed by colony growth on plates, which remains the gold standard for assessment of cell viability. Alternative approaches, such as cell staining, offer the potential of defining viable cells by visible fluorescence of intact cell membranes but have their own shortcomings, including nonspecific binding39 and the potential for false association with viability.40
It is worth noting that a single strain each was selected for study and that other strains may show different morphologic, physiologic, and phenotypic properties. Nevertheless, in an earlier study, E. faecalis showed similar starvation survival kinetics when 2 strains were compared9 and the present results for C. albicans are consistent with previous studies that demonstrate long-term survival of the species in water.29-31
In the root-filled canal, microorganisms may be interred within dentin, filling material, adjacent voids, or anatomic ramifications separate from the main root canal. Nutrient availability at these sites is likely to vary from substrate replete to complete starvation. As shown recently, some prevalent endodontic pathogens cannot survive starvation, and the prospects of survival depend on a higher level of serum as a nutritional source.41 Whether individual species will endure and have the possibility to participate in posttreatment disease depends on many factors, but the present findings, in conjunction with other studies,9,41 show that cell numbers, starvation-survival capacity of the species, and availability of even low amounts of serum will likely influence their fate.
In conclusion, this study has shown that C. albicans exhibits starvation survival behavior similar to E. faecalis. Both species are capable of starvation survival for >6 months and are able to use low levels of serum for growth. These characteristics are conducive to species survival and contribution to posttreatment apical periodontitis.
The authors thank Dr. Sally Turner for research advice and Prof. Göran Sundqvist for valuable criticism.
REFERENCES
1. Sundqvist G. Bacteriological studies of necrotic dental pulps. Umeå University odontological dissertations no. 7. Umeå (Sweden): Umeå University; 1976.
2. Sundqvist G. Taxonomy, ecology, and pathogenicity of the root canal flora. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1994;78:522-30.
3. Sundqvist G, Figdor D, Persson S, Sjögren U. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1998;85:86-93.
4. Molander A, Reit C, Dahlén G, Kvist T. Microbiological status of root-filled teeth with apical periodontitis. Int Endod J 1998;31:1-7.
5. Hancock HH, III, Sigurdsson A, Trope M, Moiseiwitsch J. Bacteria isolated after unsuccessful endodontic treatment in a North American population. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2001;91:579-86.
6. Peciuliene V, Reynaud AH, Balciuniene I, Haapasalo M. Isolation of yeasts and enteric bacteria in root-filled teeth with chronic apical periodontitis. Int Endod J 2001;34:429-34.
7. Pinheiro ET, Gomes BP, Ferraz CC, Sousa EL, Teixeira FB, Souza-Filho FJ. Microorganisms from canals of root-filled teeth with periapical lesions. Int Endod J 2003;36:1-11.
8. Peciuliene V, Balciuniene I, Eriksen HM, Haapasalo M. Isolation of Enterococcus faecalis in previously root-filled canals in a Lithuanian population. J Endod 2000;26:593-5.
9. Figdor D, Davies JK, Sundqvist G. Starvation survival, growth and recovery of Enterococcus faecalis in human serum. Oral Microbiol Immunol 2003;18:234-9.
10. Arendorf TM, Walker DM. The prevalence and intra-oral distribution of Candida albicans in man. Arch Oral Biol 1980;25: 1-10.
11. Egan MW, Spratt DA, Ng YL, Lam JM, Moles DR, Gulabivala K. Prevalence of yeasts in saliva and root canals of teeth associated with apical periodontitis. Int Endod J 2002;35:321-9.
12. Lana MA, Ribeiro-Sobrinho AP, Stehling R, Garcia GD, Silva BK, Hamdan JS, et al. Microorganisms isolated from root canals presenting necrotic pulp and their drug susceptibility in vitro. Oral Microbiol Immunol 2001;16:100-5.
13. Möller ÅJR. Microbiological examination of root canals and periapical tissues of human teeth. Methodological studies. Odontol Tidsk 1966;74:(Suppl):1-380.
14. Siqueira JF Jr, Rôças IN, Moraes SR, Santos KR. Direct amplification of rRNA gene sequences for identification of selected oral pathogens in root canal infections. Int Endod J 2002;35: 345-51.
15. Siqueira JF Jr, Rôças IN, Lopes HP. Patterns of microbial colonization in primary root canal infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002;93:174-8.
16. Baumgartner JC, Watts CM, Xia T. Occurrence of Candida albicans in infections of endodontic origin. J Endod 2000; 26:695-8.
17. Cheung GS, Ho MW. Microbial flora of root canal-treated teeth associated with asymptomatic periapical radiolucent lesions. Oral Microbiol Immunol 2001;16:332-7.
18. Waltimo TM, Sirén EK, Torkko HL, Olsen I, Haapasalo MP. Fungi in therapy-resistant apical periodontitis. Int Endod J 1997;30:96-101.
19. Siqueira JF Jr, Rôças IN. Polymerase chain reaction-based analysis of microorganisms associated with failed endodontic treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2004;97:85-94.
20. Rôças IN, Hülsmann M, Siqueira JF, Jr. Microorganisms in root canal-treated teeth from a German population. J Endod 2008; 34:926-31.
21. Waltimo TM, Sen BH, Meurman JH, Ørstavik D, Haapasalo MP. Yeasts in apical periodontitis. Crit Rev Oral Biol Med 2003; 14:128-37.
22. Siqueira JF, Jr, Sen BH. Fungi in endodontic infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2004;97: 632-41.
23. McCullough MJ, Ross BC, Reade PC. Candida albicans: a review of its history, taxonomy, epidemiology, virulence attributes, and methods of strain differentiation. Int J Oral Maxillofac Surg 1996;25:136-44.
24. Calderone R, Suzuki S, Cannon R, Cho T, Boyd D, Calera J, et al. Candida albicans: adherence, signaling and virulence. Med Mycol 2000;38 Suppl 1:125-37.
25. Jacob AE, Hobbs SJ. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol 1974;117:360-72.
26. Giard JC, Hartke A, Flahaut S, Boutibonnes P, Auffray Y. Glucose starvation response in Enterococcus faecalis JH2-2: survival and protein analysis. Res Microbiol 1997;148:27-35.
27. Sedgley CM, Lennan SL, Appelbe OK. Survival of Enterococcus faecalis in root canals ex vivo. Int Endod J 2005;38:735-42.
28. Castellani A. The viability of some pathogenic fungi in sterile distilled water. J Trop Med Hyg 1939;42:225-6.
29. Hartung de Capriles C, Mata S, Middelveen M. Preservation of fungi in water (Castellani): 20 years. Mycopathologia 1989;106: 73-9.
30. McGinnis MR, Padhye AA, Ajello L. Storage of stock cultures of filamentous fungi, yeasts, and some aerobic actinomycetes in sterile distilled water. Appl Microbiol 1974;28:218-22.
31. Odds FC. Long-term laboratory preservation of pathogenic yeasts in water. J Med Vet Mycol 1991;29:413-5.
32. del Mar Lleò M, Pierobon S, Tafi MC, Signoretto C, Canepari P. mRNA detection by reverse transcription-PCR for monitoring viability over time in an Enterococcus faecalis viable but non-culturable population maintained in a laboratory microcosm. Appl Environ Microbiol 2000;66:4564-7.
33. Nair PNR, Sjögren U, Krey G, Kahnberg K-E, Sundqvist G. Intraradicular bacteria and fungi in root-filled, asymptomatic human teeth with therapy-resistant periapical lesions: a long-term light and electron microscopic follow-up study. J Endod 1990;16:580-8.
34. Nair PNR. On the causes of persistent apical periodontitis: a review. Int Endod J 2006;39:249-81.
35. Pendrak ML, Yan SS, Roberts DD. Sensing the host environment: recognition of hemoglobin by the pathogenic yeast Candida albicans. Arch Biochem Biophys 2004;426:148-56.
36. Şen BH, Safavi KE, Spångberg LS. Growth patterns of Candida albicans in relation to radicular dentin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1997;84:68-73.
37. Brown DH Jr, Giusani AD, Chen X, Kumamoto CA. Filamentous growth of Candida albicans in response to physical environmental cues and its regulation by the unique CZF1 gene. Mol Microbiol 1999;34:651-62.
38. Tripathi G, Wiltshire C, Macaskill S, Tournu H, Budge S, Brown AJ. Gcn4 co-ordinates morphogenetic and metabolic responses to amino acid starvation in Candida albicans. EMBO J 2002;21:5448-56.
39. Biggerstaff JP, Le Puil M, Weidow BL, Prater J, Glass K, Radosevich M, et al. New methodology for viability testing in environmental samples. Mol Cell Probes 2006;20:141-6.
40. Renye JA, Jr, Piggot PJ, Daneo-Moore L, Buttaro BA. Persistence of Streptococcus mutans in stationary-phase batch cultures and biofilms. Appl Environ Microbiol 2004;70:6181-7.
41. Brundin M, Figdor D, Sundqvist G, Sjögren U. Starvation response and growth in serum of Fusobacterium nucleatum, Peptostreptococcus anaerobius, Prevotella intermedia, and Pseudoramibacter alactolyticus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009;108:129-34.
Reprint requests:
Dr. David Figdor
517 St Kilda Road
Melbourne, VIC 3004
Australia
Supported by the Australian Society of Endodontology and Melbourne Dental School, University of Melbourne.
aMelbourne Dental School, University of Melbourne.
bDepartment of Microbiology, Monash University.
Received for publication Jan 15, 2010; returned for revision Mar 6, 2010; accepted for publication Mar 9, 2010.
1079-2104/$ - see front matter
© 2010 Mosby, Inc. All rights reserved.
doi:10.1016/j.tripleo.2010.03.007