| 0099-2399/93/1906-0302/$03.00/0 | |
| JOURNAL OF ENDODONTICS | Printed in U.S.A. |
| Copyright © 1993 by The American Association of Endodontists | VOL. 19, NO. 6, JUNE 1993 |
pH Changes in Root Dentin over a 4-Week Period following Root Canal Dressing with Calcium Hydroxide
Alan Nerwich, BDS, MDSc, David Figdor, MDSc, FRACDS, Dip Endo, and Harold H. Messer, MDSc, PhD
Root canals in extracted human teeth were cleaned and shaped and subsequently dressed with a calcium hydroxide root canal dressing. pH Changes in the root dentin were measured over a 4-wk period with microelectrodes in small cavities at apical and cervical levels in inner and outer dentin. The pH increased within hours in the inner dentin, peaking at pH 10.8 cervically and 9.7 apically. However, 1 to 7 days elapsed before the pH began to rise in the outer root dentin, reaching peak levels of pH 9.3 cervically and 9.0 apically after 2 to 3 wk. The results show that hydroxyl ions derived from a calcium hydroxide dressing do diffuse through root dentin. They diffuse faster and reach higher levels cervically than apically. Surface pH measurements showed that hydroxyl ions do not diffuse in more than a minor way through the intact root surface.
Calcium hydroxide has been used in endodontic therapy since 1920 when Hermann first described its use in the treatment of nonvital teeth. In modern endodontic treatment, it is most commonly utilized as an intracanal dressing. The role of calcium hydroxide in endodontics includes its ability to induce hard tissue formation (1), its ability to cause intratubular occlusion (2), its antibacterial actions (3), and its tissue dissolving capability (4).
For calcium hydroxide to act effectively as an intracanal dressing, the hydroxyl ion must be able to diffuse through the dentin. It might be expected that this would occur in a manner similar to water, since diffusion through dentin is primarily determined by molecular weight (5). Several studies have attempted to measure diffusion of hydroxyl ions through dentin using a variety of experimental approaches, including pH-indicating solutions or papers (6), pH measurement of ground dentin (7), and pH values of the surrounding medium (8). Tronstad et al. (6) examined histological sections of monkey teeth 1 month after placement of a calcium hydroxide root canal dressing and, using indicator solutions, found that there was a pH gradient with high values around the root canal decreasing toward the peripheral dentin. The pH of the cementum remained unchanged but in resorption areas, where cementum was not present, the increased pH extended to the dentin surface. In a study related to the action of calcium hydroxide in cervical resorption (9), calcium hydroxide was placed in the cervical part of root canals previously filled with bleaching agents. A pH reversal from a slightly acid level to a slightly alkaline level was demonstrated with pH electrodes and alkacid test papers. In another study (8), pH changes were measured in distilled water surrounding teeth filled with calcium hydroxide. Only very small changes in pH levels were detected up to 10 days.
Recently, Wang and Hume (7) measured hydroxyl ion diffusion across the dentin between an occlusal cavity containing calcium hydroxide and a saline-filled pulp chamber at 16 days, using a pH meter. By taking ground dentin (subsequently mixed with saline) from various depths, they demonstrated a gradient of pH values from the cavity layer decreasing to the middle and pulpal layers, indicating a slow movement of the hydroxyl ion through the dentin. Importantly, they showed in a series of experiments that dentin has the capacity to buffer hydroxyl ions as these ions diffuse through dentin.
Although diffusion of the hydroxyl ion through dentin clearly does occur, the diffusion dynamics of calcium hydroxide through root dentin has not been studied accurately over time. The purpose of this study was, therefore, to investigate pH changes over a period of 4 wk after application of a calcium hydroxide dressing; in particular the maximum pH and the time taken to reach this maximum at the inner and outer dentin as well as on the root surface, at cervical and apical levels of the tooth root. pH Microelectrodes were used to precisely measure the pH changes in root dentin in vitro.
MATERIALS AND METHODS
Selection, Preparation, and Storage of Teeth
Twelve extracted human permanent teeth with single canals were collected (nine canines, one maxillary central incisor, and two mandibular premolars). The teeth had been removed from patients under 35 yr of age. They were divided into an experimental group consisting of 10 teeth and a control group of 2 teeth. Adherent tissue was removed gently with forceps, but the root surface was otherwise left intact. Care was taken not to damage cementum. Prior to experimentation, the teeth were stored at 4°C in a solution of 0.05% sodium azide in unbuffered saline.
Endodontic Preparation of Root Canals
The root canals were prepared with a rubber dam in place so as not to contaminate the root surface with sodium hypochlorite. All teeth were cleaned and shaped to a minimum of a size 40 master file, 1 mm from the anatomical apex. The root canals were flared using a step-back technique followed by the use of Gates Glidden drills. Irrigation during cleaning and shaping was carried out with 5 to 10 ml of 1% sodium hypochlorite. The canals were flushed with 1 ml of 17% EDTA which was left in place for 5 min to remove smear layer at the termination of instrumentation. The canals were then given a final flush with 5 ml of 1% sodium hypochlorite. The patency of the apical foramen was checked with a size 15 K file.
Root Preparation
Cavities were drilled at two levels on the root surface of all teeth; at the cervical level (3 to 5 mm apical to the cementoenamel junction) and an apical level (3 to 5 mm cervical to the apex) using an end cutting high-speed bur followed by a 1.75-mm diameter drill (Parapost; Whaledent, New York, NY). The distance from the root canal wall at each level was estimated from proximal radiographs. Two cavities were drilled at the apical and cervical levels. The first, inner dentin cavity, was cut to a distance of about 1.0 mm from the canal wall and the second, outer dentin cavity, to a depth of about 0.5 mm from the root surface. The thickness of dentin between the root canal wall and the cavities was measured with an electronic caliper after sectioning the teeth at the completion of experiments. The mean values and standard deviations were: apical inner, 0.97 ± 0.21 mm; apical outer, 1.66 ± 0.31 mm; cervical inner, 1.10 ± 0.10 mm; cervical outer, 2.54 ± 0.32 mm. Smear layer in these cavities was removed with 17% EDTA solution. Scanning electron microscopy of similar root surface cavities showed that a 3- to 4-min application of EDTA efficiently removed the smear layer without opening up the dentinal tubules.
Placement of Calcium Hydroxide in Canals
The 10 experimental teeth were filled by injecting a calcium hydroxide intracanal dressing (Calasept: Scania Dental, Knivsta, Sweden). Calasept is an aqueous suspension of calcium hydroxide, 100 g of which contains 56 g of calcium hydroxide, 0.35 g of sodium chloride, 8 mg of potassium chloride, 8 mg of calcium chloride, 4 mg of sodium bicarbonate and water, with a pH of 12.5. The control teeth were filled with isotonic saline. The access cavities were filled with at least 4-mm deep seal of Cavit (Espe; GmbH, Seefeld/Oberbay, Germany).
Determination of pH
The pH of the dentin in each location was determined following application of calcium hydroxide at 0, 3, 6, 12, 24, and 48 h and at 7, 21, and 28 days. These intervals were based on a pilot study. Each experimental tooth was placed in 100 ml of isotonic unbuffered saline and stored at 37°C. Control teeth were placed in the same container as an experimental tooth. The saline was replaced if the pH rose above 7.4. At every time interval, each tooth was removed from the saline and rinsed in distilled water to remove saline residue. The cavities were then blotted dry and 2 µl of distilled water were placed in each cavity. The pH of the distilled water in each cavity and on each tooth surface was measured using a pH microelectrode (model MI 4152; Microelectrodes Inc, Londonderry, New Hampshire). The pH microelectrode was calibrated with solutions of known pH before and after measurements at each time period.
Statistical Analysis
Each measurement series on a particular cavity was summarized by a weighted average taken over time, resulting in a value which represents the average pH over the 28 days. These values were compared using an analysis of variance model and, where appropriate, Student’s t test. The statistical method is based on an article by Matthews et al. (10).
RESULTS
When the root canals of all teeth were cleaned and shaped, the baseline pH for all dentin test cavities and the intact root surface was approximately pH 8.5. It is likely that the relatively high baseline pH was due to diffusion of sodium hypochlorite (pH 11) through dentin during canal irrigation. After the experiment commenced, the pH dropped initially in both the control and experimental teeth so that the 3-h measurements were significantly lower for cervical outer (p < 0.01), apical inner (p < 0.01), and apical outer (p = 0.01) dentin. For the cervical inner dentin, the initial drop was not significant. Thereafter, the pH levels of the control teeth remained within a range of 7.6 to 8.3 throughout the duration of the study.
For the experimental teeth, the cervical inner dentin was the most rapid to rise. The rise in pH began in the first 6 h, peaked at 10.8 after 24 h and settled to a stable pH value of just above 10. Apically, the pH of the inner dentin began to rise between 6 and 12 h and continued to rise steadily, reaching a plateau of approximately 9.5 after 2 wk. The pH of the cervical outer dentin began to increase between 3 and 7 days, rising to just over 9 at 2 wk and reached a stable level of about 9.3 after 3 wk. In the apical outer dentin, although the pH began rising earlier (after 24 h), the maximum pH level was lower, reaching just under 9 at 2 wk. The pH values on the intact root surface reached 8.4 at 28 days.
The mean pH values of the treatment teeth are depicted graphically in Fig. 1. The means of the weighted average pH values over the 28-day test period are shown in Table 1.
The data were analyzed using an analysis of variance model. This showed a highly significant difference between treatment and control teeth (p = 0.000) and highly significant differences between position on the tooth (p = 0.003) and between inner and outer dentin (p = 0.000). The cervical inner cavity showed a significant difference between treatment and control teeth (p < 0.01), as did the apical inner cavity (p < 0.01), the cervical outer cavity (p < 0.05), and the apical outer cavity (p < 0.05). There was no significant difference between the pH values for the experimental and control values on the root surface. There was a highly significant interaction term between location and depth of cavity (p = 0.007), indicating that the effects of the position of the cavity—apical or cervical—and the depth of the cavity were not simply additive.
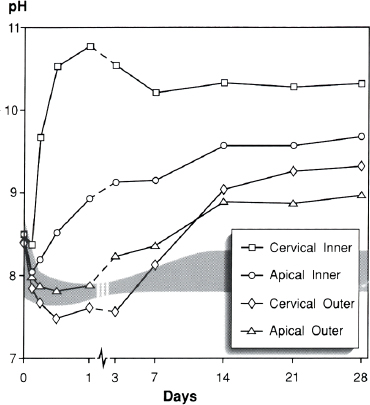
FIG 1. pH Changes in root dentin over 28 days following root canal dressing with calcium hydroxide. The gray shaded area represents the range for pH values of the control teeth (inner, outer, cervical, and apical dentin).
TABLE 1. Mean of weighted average pH values over 28 days
| Control | Experimental | |
|
Apical outer |
8.02 (0.29)* | 8.85 (0.13) |
|
Apical inner |
8.02 (0.29) | 9.39 (0.13) |
|
Cervical outer |
8.01 (0.29) | 8.69 (0.13) |
|
Cervical inner |
7.83 (0.29) | 10.32 (0.13) |
|
Surface |
7.9 (0.48) | 8.01 (0.21) |
* Numbers in parentheses represent standard error values.
DISCUSSION
Calcium hydroxide is a routinely used dressing in endodontic therapy, yet relatively little is known about the diffusion dynamics of this medicament. The purpose of this investigation was to determine how hydroxyl ions diffuse through root dentin over time by measuring the rapidity, magnitude, and duration of increase in pH at the inner and outer dentin, at both the apical and cervical levels and surface of the tooth root.
After calcium hydroxide paste was placed into the root canal, the pH of the cervical inner dentin rose rapidly over 3 to 6 h, reaching a maximum of 10.8 in just 24 h. This suggests that hydroxyl ions, like other small molecules, were able to diffuse readily through the inner 1-mm layer of permeable cervical dentin. Hydroxyl ions diffused less readily, however, in apical dentin. Here, the thickness of the dentin (canal wall to cavity) was almost identical, but the rise in pH began later (after 12 to 24 h) and took longer to peak, reaching a lower plateau of approximately 9.5 after 2 wk. Since the distance for the hydroxyl ions to traverse to the point of measurement was almost the same for the apical as the cervical cavity (about 1 mm), this implies that a difference exists between the permeability of apical and cervical dentin. Evidence in support of this comes from Marion et al. (11) who found the diameter of the apical tubules to be less then the cervical tubules, and Carrigan et al. (12) who found that there are fewer tubules in the apical region of the root than in the cervical part. Since diffusive movement through dentin occurs via the dentinal tubules and is directly proportional to dentinal tubule density and the square of the tubule radius (5), these factors affect the diffusion of hydroxyl ions.
In the apical outer dentin, 1.66 mm from the root canal wall, the pH began rising after 1 to 3 days, reaching a value of just under 9 at 2 wk. That the pH took so much longer to rise and reach a maximum might initially be considered surprising when the hydroxyl ions had only 0.66 mm further than the inner cavity to traverse. However, recent evidence from Wang and Hume (7) shows that dentin has a strong buffering property. This means that the hydroxyl ions leaving the canal space must overcome the buffering effect of the hydroxyapatite before being able to diffuse further toward peripheral dentin. Additionally, hydroxyl ions may be slowed by a decreasing tubule diameter as the cementum is approached.
The pH of the cervical outer dentin was the last to rise, commencing between the third and seventh day and increasing to 9.04 at 2 wk and reaching a stable level of 9.26 after 3 wk. This prolonged rise suggests that the hydroxyl ions were buffered as they traversed the 2.54 mm to the cervical outer dentin cavity. Eventually, the pH in this cavity rose higher than the apical outer cavity. This indicates that once the dentin buffering reached its maximum capacity, the greater permeability of the cervical dentinal tubules allowed more hydroxyl ions to reach this cavity.
The surface pH measurements were slightly higher than those of the controls, although not significantly so, at 4 wk, indicating that the hydroxyl ion does not diffuse in more than a minor way through the intact root surface.
Our results compare favorably with previous work. Indicator solutions have been used to assess the pH of dentin 1 month after calcium hydroxide dressing was placed in the canal (6), showing that inner dentin was in the range of 8.0 to 11.1 and the pH of the outer dentin was in the range of 7.4 to 9.6. Wang and Hume (7) placed calcium hydroxide paste in a coronal cavity and after 16 days detected a similar pH gradient. The cavity layer pH value was 9.66, the middle and pulpal layers were 9.07 and 8.88, respectively. These findings are similar to our results for root dentin at 14 days. Fuss et al. (8) detected only very minor changes at 10 days in the pH of 40 ml of distilled water around teeth filled with calcium hydroxide and with cervical cementum removed. They concluded that the hydroxyl ion does not diffuse through dentin. In our experiments, we also found only a slight increase in pH between 1 and 2 wk in 100 ml of saline. However, subtle changes in the pH of a large volume of surrounding water do not accurately reflect the capacity for hydroxyl ion diffusion; our results clearly show that these ions do permeate through dentin.
The peak pH levels of inner dentin were higher than those of the corresponding outer dentin. There are three likely reasons for this observation. First, the number of dentinal tubules communicating with the outer test cavity were probably fewer than those in the inner cavity. Dentinal tubules radiate outward from the root canal wall to the peripheral root surface, so the density of tubules decreases as the distance from the root canal increases. In these experiments the diameter of the test cavities was the same in the inner and outer dentin, thus there would be fewer tubules in the outer dentin cavities. Second, tubule diameter diminishes in size as the cementum is approached (13), so the outer cavities would have smaller tubules. Third, solute concentration dissipates over distance (5), so that less hydroxyl ions reach the outer dentin.
Diffusion of the hydroxyl ion through dentin can be explained by dentinal permeability as well as interactions between dentin and hydroxyl ions. The permeability of dentin (specifically diffusive transport) is governed largely by tubular anatomy—their density, diameter, and length as well as features of the solute such as size and charge (5). Hydroxyl ions may also be affected by buffering, adsorption, and charge of the dentin. Buffering may occur when proton donors (such as H3PO4– H2CO3, and HCO3–) in the hydrated layer of hydroxyapatite (14) furnish additional protons to keep the pH unchanged. Hydroxyl ions may also be adsorbed into the hydrated layer, thus slowing their diffusion along the dentinal tubule. Dentin has a net charge which may vary under different conditions (15) but which may also influence the passage of hydroxyl ions.
It is useful to consider how diffusion mechanisms affect the passage of hydroxyl ions through dentin. Initially, as hydroxyl ions diffuse into the circumpulpal dentin, the permeability of dentin is the prime factor since there is insufficient bulk of dentin to significantly buffer or adsorb the ions. As the hydroxyl ions continue to traverse through dentin, the tubule diameter diminishes and buffering and related properties become more dominant as the dentin bulk increases. The hydroxyl ions must overcome these latter effects before diffusing further through dentin. Eventually, after 2 to 3 wk, the whole thickness of dentin is saturated with hydroxyl ions as indicated by the detection of a raised pH at the outer dentin surface. It is then the permeability of dentin that dictates the final diffusion of the hydroxyl ion.
It has been demonstrated that calcium hydroxide is an efficient and reliable antibacterial dressing for infected root canals (3, 16). In vitro experiments have shown that when endodontic bacteria were added to a suspension of calcium hydroxide, 26 of 27 strains were killed in less than 6 min (3). However, a 10-min application of calcium hydroxide in vivo was ineffective in sterilizing root canals (16). Our results explain why this might be so. Calcium hydroxide clearly kills bacteria that are directly in contact; however, many bacteria are known to be located in the circumpulpal dentin (17). In the first minutes, and indeed days, hydroxyl ions do not diffuse sufficiently into dentin to kill these bacteria. Our results show that a duration of at least 1 wk with calcium hydroxide is required to raise the pH of the inner dentin to 9.0, a level at which many bacteria do not grow (18).
In a resorptive lesion following trauma, the root surface is damaged by activated odontoclasts in areas where cementum has been denuded. The test cavities we prepared on the root surface for pH measurement are similar to those induced pathologically in a resorptive lesion. Hammarström et al. (19) showed in experimentally induced resorption that a calcium hydroxide intracanal dressing initially caused necrosis of the resorbing cells, a finding recently supported by Lengheden et al. (20). They (19, 20) postulated that the diffusion of the hydroxyl ion through dentin caused these effects. Our results confirm that hydroxyl ions do diffuse through dentin, potentially enabling them to reach a site of resorption.
The results show that hydroxyl ions derived from a calcium hydroxide intracanal dressing diffuse in a matter of hours into the inner root dentin but require 1 to 7 days to reach the outer root dentin and 2 to 3 wk to reach peak levels. They diffuse faster and reach higher levels cervically than apically. The permeability and buffering capacity of dentin are key factors affecting the diffusion of hydroxyl ions through root dentin. Hydroxyl ions do not diffuse significantly through the intact root surface.
This study was supported by research grants from the Australian Society of Endodontology and University of Melbourne.
We wish to thank Professor Goran Sundqvist, Sweden, for valuable criticism and Rohan Wilson and Sieglinde Jobson for laboratory assistance.
Drs. Nerwich, Figdor, and Messer are affiliated with the School of Dental Science, Faculty of Medicine, Dentistry, and Health Sciences, University of Melbourne, Melbourne, Australia. Address requests for reprints to Dr. David Figdor, Medical Centre, 517 St. Kilda Road, Melbourne 3004, Australia.
References
1. Zander HA. Reaction of the pulp to calcium hydroxide. J Dent Res 1939;6:373–9.
2. Mjör A, Furseth R. The inorganic phase of calcium hydroxide- and corticosteroid-covered dentin studied by electron microscopy. Arch Oral Biol 1968;13:755–63.
3. Byström A, Claesson R, Sundqvist G. The antibacterial effect of camphorated paramonochlorophenol, camphorated phenol and calcium hydroxide in the treatment of infected root canals. Endod Dent Traumatol 1985;1:170–5.
4. Hasselgren G, Olsson B, Cvek M. Effects of calcium hydroxide and sodium hypochlorite on the dissolution of necrotic porcine muscle tissue. J Endodon 1988;14:125–7.
5. Pashley DH. Dentine permeability: theory and practice. In: Spångberg LSW, ed. Experimental endodontics. Boca Raton, FL: CRC Press, 1990:19–49.
6. Trondstad L, Andreasen JO, Hasselgren G, Kristerson L, Riis I. pH changes in dental tissues after root canal filling with calcium hydroxide. J Endodon 1981;7:17–21.
7. Wang JD, Hume WR. Diffusion of hydrogen ion and hydroxyl ion from various sources through dentin. Int Endod J 1988;21:17–26.
8. Fuss Z, Szajkis S, Tagger M. Tubular permeability to calcium hydroxide and to bleaching agents. J Endodon 1989;15:362–4.
9. Kehoe JC. pH Reversal following in vitro bleaching of pulpless teeth. J Endodon 1987;13:6–9.
10. Matthews JNS, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. Br Med J 1990;300:230–5.
11. Marion D, Jean A, Hamel H, Kerebel L, Kerebel B. Scanning electron microscopic study of odontoblasts and circumpulpal dentin in a human tooth. Oral Surg Oral Med Oral Pathol 1991;72:473–8.
12. Carrigan PJ, Morse DR, Furst ML, Sinai IH. A scanning electron microscopic evaluation of human dentinal tubules according to age and location. J Endodon 1984;10:359–63.
13. Tidmarsh BG, Arrowsmith MG. Dentinal tubules at the root ends of apicected teeth: a scanning electron microscopic study. Int Endod J 1989;22:184–9.
14. Jenkins GN. The physiology and biochemistry of the mouth. 4th ed. Oxford: Blackwell Scientific Publications, 1978:54–112.
15. Weerkamp AH, Uyen HM, Busscher HJ. Effect of zeta potential and surface energy on bacterial adhesion to uncoated and saliva coated human enamel and dentin. J Dent Res 1988;67:1483–7.
16. Sjögren U, Figdor D, Spångberg L, Sundqvist G. The antimicrobial effect of calcium hydroxide as a short-term intracanal dressing. Int Endod J 1991;24:119–25.
17. Shovelton DS. The presence and distribution of microorganisms within non-vital teeth. Br Dent J 1964;117:101–7.
18. Mitscherlich E, Marth EH. Microbial survival in the environment. Berlin: Springer-Verlag, 1984:563–73.
19. Hammarström L, Blomlöf L, Feiglin B, Lindskog S. Effect of calcium hydroxide treatment on periodontal repair and root resorption. Endod Dent Traumatol 1986;2:184–9.
20. Lengheden A, Blomlöf L, Lindskog S. Effect of delayed calcium hydroxide treatment on periodontal healing in contaminated replanted teeth. Scand J Dent Res 1991;99:147–53.
You Might Be Interested to Know
A newer development in heroin addiction is called “chasing the dragon” which is inhaling sublimated heroin which has been heated on tin foil. Before 1970 (Br Med J 304:1222) over 75% of persons in London “initiated” into heroin use were hooked by an intravenous injection. Today, over 90% of first users join the dismal crew of users by “chasing the dragon.” This development may be of importance relative to disease transmission through infected needles. Comforting to know that becoming addicted is less hazardous than it used to be. Ah, brave new world.
Wallace Sturr