Persistent periapical radiolucencies of root-filled human teeth, failed endodontic treatments, and periapical scars
P. N. Ramachandran Nair, BVSc, DVM, PhD,a Ulf Sjögren, DDS, PhD,b David Figdor, BDSc, MDSc, FRCDS, Dip Endo,c and Göran Sundqvist, DDS, PhD,b Zurich, Switzerland, Umeå, Sweden, and Melbourne, Australia
UNIVERSITY OF ZURICH, UMEÅ UNIVERSITY, AND UNIVERSITY OF MELBOURNE
Objective. This report describes 6 cases that demonstrate persistent periapical radiolucent lesions after conventional root canal treatment.
Study design. Six teeth that had conventional root canal treatment or re-treatment with nonresolving periapical radiolucencies underwent periapical surgery. Biopsies were obtained and analyzed descriptively by correlative light and transmission electron microscopy for general features and microbial findings.
Results. Three findings were identified: periapical lesions with persisting infection in the apical root canal system (2 cases); a cyst (1 case); and periapical healing by scar tissue formation (2 cases).
Conclusions. These results confirm previous observations that associated factors in the failure of endodontic treatment include persistent intraradicular infection and periapical cysts. In addition, unresolved periapical radiolucencies may occasionally be due to healing by scar tissue, which may be mistaken as a sign of failed endodontic treatment.
(Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999;87:617-27)
Periapical bone resorption, which on a radiograph can be identified as radiolucency, is an important diagnostic feature of apical periodontitis. Because intraradicular microorganisms are usually associated with and implicated as the primary etiologic agents of apical periodontitis,1-4 root canal treatment is aimed at eliminating infection; obturation follows. When the treatment is able to be done properly, healing of the periapical lesion usually occurs with osseous regeneration, which is characterized by gradual reduction and resolution of the radiolucency on subsequent follow-up radiographs.5-14
It is generally acknowledged that in most cases in which endodontic treatment fails, the failure occurs when treatment procedures have not met a satisfactory standard for control and elimination of infection. Common problems that may lead to endodontic failure include inadequate aseptic control, poor access cavity design, missed canals, inadequate instrumentation, and leaking temporary or permanent fillings.15 Even when the highest standards are met and the most careful procedures are followed, failures still occur, inasmuch as there are root canal regions that cannot adequately be debrided and obturated wifh existing instruments, materials, and techniques.
The reasons for persistence of radiolucencies after conventional root canal treatment in well-treated cases are not fully understood. Previous investigations of periapical biopsy material have been limited by the use of unsuitable specimens, inappropriate methods, and criteria of analysis that failed to yield relevant etiologic information.16-20 Examples of procedural limitations in situations in which relevant detail may be missed include the following: light microscopy without correlative electron microscopic analysis; evaluation of random rather than serial sections; paraffin-embedding rather than resin-embedding of biopsies; and assignment of overly broad criteria, such as “bacteria and/or debris,” that can encompass many potential etiologic factors.
Recent studies that take into account appropriate case selection and methods have begun to identify some factors that may contribute to the perpetuation of peri-apical radiolucencies after root canal treatment. Factors thus far identified include the following: intraradicular infection persisting in the apical root canals of root-filled teeth21; extraradicular infection, generally in the form of periapical actinomycosis22; extruded root canal filling23 or other materials24-27 that cause foreign body reactions; and true cysts,28 especially with massive accumulation of cholesterol crystals in periapical lesions.28,29
This report presents 6 cases that demonstrated failure of bone to heal after endodontic treatment. Teeth that had been treated by endodontic therapy and had unresolved periapical radiolucencies on follow-up underwent surgery. Biopsy material was removed, and the tissue was analyzed by correlative light and transmission electron microscopy (TEM).
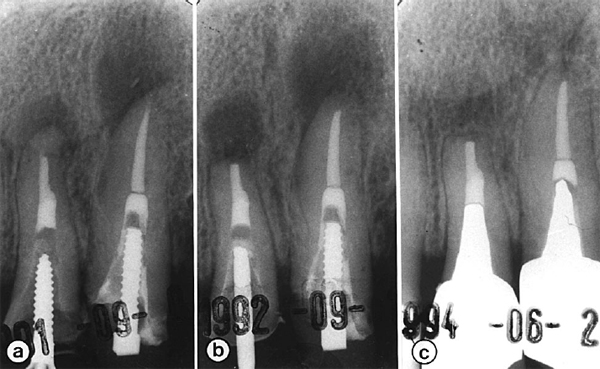
Fig 1. Successful surgery with failed endodontic re-treatment. Preoperative radiograph (a) shows failed retreatment of both teeth. Postoperative radiographs taken immediately after surgery (b) and 18 months later (c) reveal bone healing.
MATERIAL AND METHODS
Clinical material
Six biopsies were obtained from teeth with periapical radiolucencies that persisted after root canal treatment (Table I). A specialist at the Endodontic Clinic, University of Umeå, Sweden, treated all of the teeth. Two biopsies (cases GRS-17 and GRS-18) were obtained from teeth that had root canal treatment in a single appointment.13 Four biopsies (cases GRS-14, GRS-15, GRS-19, and GRS-20) were derived from teeth that had re-treatment after the earlier root filling had been unsuccessful.14 The clinical, radiographic, microbiologic, and endodontic procedures have previously been described in detail.13,14 In brief, single-rooted teeth were treated at a single appointment; all teeth had necrotic pulps and radiographic evidence of apical periodontitis. The root canals were irrigated with 0.5% sodium hypochlorite solution and instrumented by manual filing and ultrasonics. After instrumentation, a bacterial sample was taken and the teeth were obturated through use of the rosin chloroform gutta-percha technique at the same appointment. Subsequent microbiologic analysis showed that some canals had contained small numbers of microorganisms at the time of root filling13; case GRS-17, examined in this study, was from that group (Table I).
Teeth that had re-treatment14 were initially instrumented with burs and files to remove the root filling material without chemical solvents. No antibacterial irrigation was used at the first appointment, and a postinstrumentation bacterial sample was taken. To increase the possibility of detecting bacteria, the canals were left empty but sealed with zinc oxide-eugenol cement for 7 days before a second bacteriologic sampling. Canals were then instrumented, irrigated with 0.5% sodium hypochlorite, and dressed with calcium hydroxide for at least 1 week. At the third appointment, a postmedication sample was taken and the canal was obturated with rosin chloroform and gutta-percha.
Patients were recalled annually for clinical and radiographic examination for up to 5 years. Periapical surgery with stringent antiseptic measures was performed if the periapical radiolucencies increased in size or remained unchanged. Special care was taken during each operation to keep the periapical lesion as intact as possible (along with a portion of the root tip) when it was removed, although in one case it was not possible to do so.
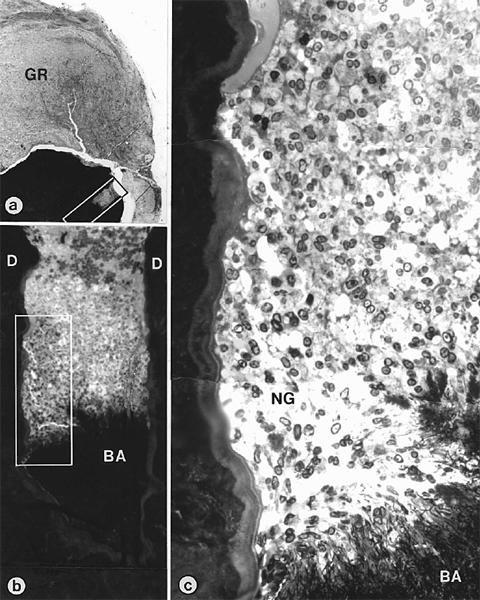
Fig 2. Intraradicular bacteria in a case of failed, properly restored, and correctly sealed re-treatment of Fig 1. Photomicrograph (a) shows axial section through block biopsy removed from radiolucent area around root-tip of maxillary left lateral incisor shown in Fig 1, b. Composites (b, c) are magnified views of demarcated areas in Fig 2, a and Fig 2, b, respectively. Note bacterial mass (BA in b and c) in root canal and defensive wall of neutrophils (NG). GR, Granuloma; D, dentin. (Original magnifications: a, ×22; b, ×150; c, ×850)

Fig 3. Transmission electron microscopic view shows intraradicular bacterial mass of Fig 2, c. Note filamentous and branching organisms (arrowhead), which reveal gram-positive wall structure. Circular inset is magnification of arrowheaded filamentous and branching organism. D, Dentin. (Original magnification ×4000)
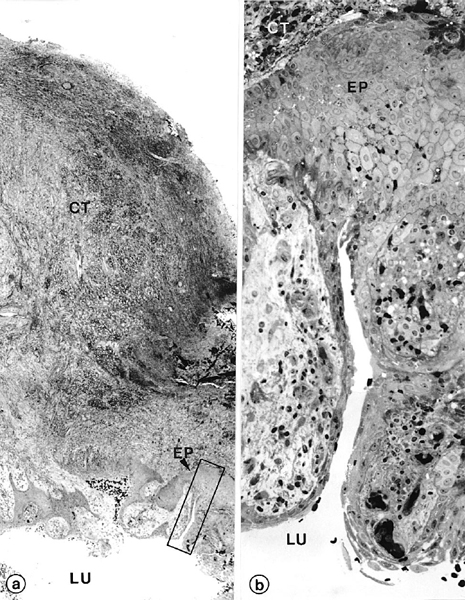
Fig 4. Wall of radicular cyst. Photomicrograph (a) shows axial section through apical biopsy removed from radiolucent area around root-tip of left maxillary canine of Fig 1, b. Demarcated area in Fig 4, a is magnified in Fig 4, b. Note empty lumen (LU) lined by distinct stratified squamous epithelium (EP) of varying thickness. Extraepithelial connective tissue (CT) is infiltrated with lymphocytes and plasma cells. (Original magnifications: a, ×52; b, ×330)
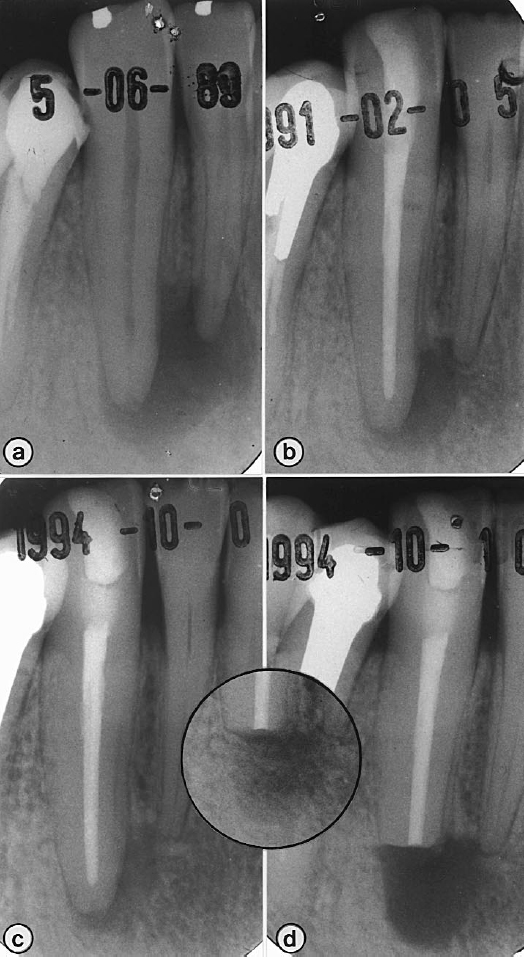
Fig 5. Preoperative radiograph (a) shows canine with periapical lesion; postoperative radiograph (b) shows same tooth after root canal treatment. Persistent radiolucency noted at 5-year follow-up (c) was removed surgically (d). Six months after surgery (inset), there is evidence of bone regeneration.
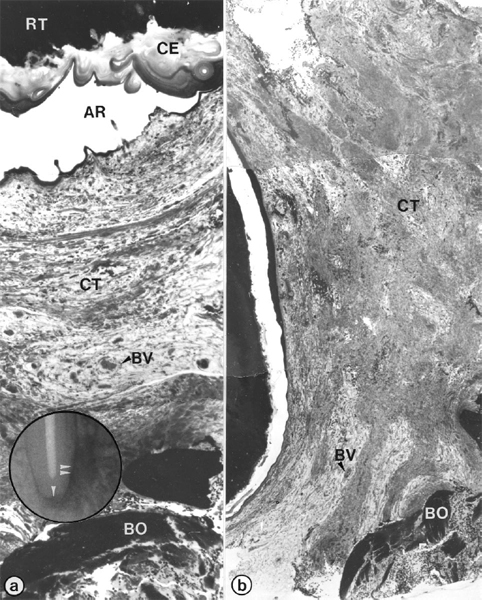
Fig 6. Periapical scar of root-filled tooth of Fig 5. Light microscopic views (a, b) show sequential axial sections in mesiodistal plane through biopsy removed from radiolucent area in Fig 5, d. Composite (a) depicts area of root-tip (RT) and subjacent apical periodontium (inset; single arrowhead); also shown (b) is mid-plane of radiolucent area (inset; double arrowhead). Note stratified acellular cementum (CE) coating of root-tip (a) and dense collagenous soft connective tissue (CT in Fig 6, a and b). AR, Artifact, BO, bone, BV, blood vessels. (Original magnifications ×52)
Table 1. Summary of clinical and follow-up data on biopsy specimens
| Case | Treatment* | Age (y) | Gender | Tooth† | Microscopy | Postsurgical radiograph |
| GRS-14 | Re-treat | 57 | F | 10 | Bacteria in root canal; granuloma | Healed |
| GRS-15 | Re-treat | 57 | F | 11 | Periapical cyst | Healed |
| GRS-17 | 1-visit | 40 | M | 27 | Periapical cyst | Healing |
| GRS-18 | 1-visit | 43 | M | 20 | Bacteria in root canal; abscess | Healed |
| GRS-19 | Re-treat | 64 | M | 7 | Granuloma | Healed |
| GRS-20 | Re-treat | 57 | F | 7 | Periapical scar | Healed |
*Root-filled by conventional re-treatment (Re-treat) or in 1 appointment (1-visit)
†10, Maxillary left second incisor; 11, maxillary left canine; 27, mandibular right canine; 20, mandibular left second premolar; 7, maxillary right second incisor.
Histopathologic findings
Immediately after surgical removal, the biopsies were fixed by immersion in half-strength Karnovsky’s fixative30 and processed as described previously.21 In brief, the root tip was decalcified for several weeks in solutions containing 0.25 mol/L ethylenediamine tetraacetic acid (Titriplex-III, Merck) and 4% glutaraldehyde supplemented with sucrose. The demineralized root tip and associated soft tissue were subdivided into 0.2- to 0.5-mm–thick slices (in either the axial or the horizontal plane), osmicated, dehydrated in ascending grades of ethanol, and embedded in Epon. Sections approximately 1 to 2 µm in thickness were prepared from each specimen block, stained in periodic acid–Schiff and methylene blue-Azure II,31 and photomicrographed by means of a Leitz-Orthoplan photomicroscope. The sections and photomicrographs were studied, and suitable sites were located on the specimen blocks for TEM examination. Such tissue sites were target-trimmed and thin-sectioned through use of an Ultracut E microtome (Reichert-Jung, Vienna, Austria). The sections were double-contrasted with lead and uranium salts32,33 and examined with a Philips-201 transmission electron microscope. If no microorganisms were found in the first series of ultrasections, the tissue blocks were step-serial sectioned and the sections were examined by means of TEM until microorganisms were encountered or the tissue specimens were exhausted through sectioning, as described previously.21
Microscopic analysis
The histologic characterization of the lesions was based on the presence or absence of microorganisms in the apical root canal and structural components of the periapical tissues. The descriptive analysis was based on the types and distribution of inflammatory cells and dense fibrous connective tissue and on the presence or absence of epithelial cells, as well as on whether the lesions had developed into cysts.
RESULTS
Of the 6 periapical biopsies investigated (Table I), 2 showed distinct bacterial infection in the root canal, 1 was a radicular cyst, 2 revealed scar-tissue healing of the periapex, and 1 was a granulomatous lesion in which no apparent reason for the persistence of radiolucency could be identified.
Periapical lesions with intraradicular infection
A bacterial mass could be clearly observed by light microscopy in the apical root canal ramifications of each of 2 specimens (Table I). One of these cases (GRS-14; Fig 1) had a necrotic pulp with a periapical lesion and was treated by means of conventional endodontic therapy. The periapical lesion increased, and the tooth was then re-treated. The periapical radiolucency persisted for 2 years without signs of healing, so periapical surgery was performed and the lesion was removed by block biopsy for microscopic analysis.
Histologically, the specimen revealed a large lesion that was well encapsulated in a collagenous connective tissue (Fig 2). The lesion contained a predominantly chronic infiltrate consisting of lymphocytes and plasma cells. In succeeding section planes, several canal ramifications were found to be clogged with filamentous microorganisms that were readily recognized by light microscopy. A dense accumulation of neutrophilic granulocytes was observed within the root canal facing the bacterial front (Fig 2). In TEM, the bacterial mass appeared to consist of only one type of aggregating, filamentous, and branching microorganism with gram-positive cell walls (Fig 3).
In the other case that showed the presence of intraradicular bacteria (GRS-18), the tooth was asymptomatic during follow-up but had failed to heal. At the time of surgery, an abscess was detected13 and a bacteriologic sample taken from the periapical area showed growth of Actinomyces odontolyticus; Propionibacterium acnes, Streptococcus, and Campylobacter species.13 The abscess was confirmed by histopathologic examination of the biopsy. The root tip contained several canal ramifications, one of which was clogged with bacteria; however, no extraradicular microbes were detected in periapical tissues by means of light and electron microscopy. Ultrastructurally, the microbial population consisted of filamentous organisms with gram-positive cell walls.
Periapical cyst
Case GRS-15 was initially treated with conventional root canal treatment. Because it did not heal, the tooth was re-treated. A microbial sample taken before obturation revealed Actinomyces israelii. Histopathologically, the biopsy revealed a large cystic lesion consisting of a distinct lumen, an epithelial cyst wall, and an extraepithelial connective tissue that blended with a collagenous capsule (Fig 4). The cyst lumen appeared empty. Because the biopsy did not include the root tip, the relationship of the cyst lumen to the root canal could not be ascertained. The lining of the cyst wall consisted of a stratified squamous epithelium of considerably varying thickness. The basal cells were located on a distinct basement membrane, which was highly irregular and formed ridges. The suprabasal cells appeared increasingly flatter toward the cyst lumen. A broad layer of connective tissue that consisted of numerous blood vessels and infiltrating cells, predominantly lymphocytes and plasma cells, surrounded the epithelial lining. Moderate aggregates of cholesterol clefts were found within the extraepithelial connective tissue wall.
Periapical scar
The radiolucent areas in the biopsies of cases GRS-17 and GRS-20 (Table I) revealed a dense, collagenous, soft connective tissue that was devoid of any signs of inflammatory reaction. The biopsy for case GRS-17 (Fig 5; mandibular right canine) is representative of the two. Preoperative radiographs showed a large radiolucency around the apices of the mandibular right second incisor and canine. The canal of the canine was sampled for microbial culturing, instrumented, and root-filled with gutta-percha in a single appointment. The case was followed radiographically for 5 years, and although the size of the radiolucency declined initially, it remained unchanged for 2 years. The root tip and lesion were surgically removed. Step-serial sectioning of the biopsy did not reveal microorganisms in the canal. Several layers of acellular cementum with distinct incremental lines covered the root tip (Fig 6). The periapical area was filled with a soft, collagenous connective tissue that consisted of waves of collagen fiber bundles running in all directions.
DISCUSSION
Cases of unresolved periapical radiolucency after conventional root canal treatment were examined. Careful histopathologic analysis of biopsy material showed that infection persisting in the apical root canal and a cystic condition of the periapical lesion may have been etiologic factors in the failure of treatment, which would be consistent with previous findings.21,28 In 2 cases, we also noted the presence of periapical scar tissue healing that had probably interfered with the regeneration of periapical bone; these cases had been radiographically interpreted as failures.
In 2 biopsies, clumps of intraradicular bacteria were observed, which is consistent with findings in previous studies21,34 that showed persistent intraradicular infection to be related to failure. The detection of intraradicular microorganisms in periapical biopsies implies that microbes were probably present when the canals were obturated. This was likely in the biopsy of case GRS-18 (Table I), which originated from a tooth that had been root-filled in a single appointment.
Microbes were also detected in the root canal of a tooth that was obturated only after a negative culture was obtained (GRS-14; Table I). Such a finding indicates that intraradicular microorganisms may escape detection by culturing; this may be due to such factors as inaccessibility of the microbes to sampling by reason of their location in remote apical ramifications and a low numeric density of organisms remaining in the canals at the time of sampling.35
Although the microbial species cannot be identified from morphologic appearance, the ultrastructure of the bacterial cell wall in these cases was characteristic of gram-positive organisms, which is in accordance with previous ultrastructural findings.21 The filamentous, branching nature of the organisms is consistent with bacteria of the genera Actinomyces and Propioni-bacterium, which had dominated the flora in a sample taken during surgery in one case.13 These are recalcitrant organisms with the capacity to establish extra-radicular actinomycoses36 or similar lesions37 that can prevent periapical healing after root canal treatment.38-41
One of the 6 specimens was a periapical cyst with a distinct lumen lined by a stratified squamous epithelium. The cystic condition of periapical lesions is a factor that may impair healing after root canal treatment. Whether a radicular cyst will heal after conventional root canal treatment has been addressed28,42 and reviewed recently.43,44 Whereas some pocket cysts42 may heal after root canal treatment, true cysts are less likely to be resolved, because they are self-sustainable.28,42-46 Whether the biopsy in case GRS-15 was a true cyst or a pocket cyst could not be addressed, because the biopsy did not include the root tip. The cystic condition of the lesion alone would be sufficient to sustain the radiolucency indefinitely, although other factors might also have been involved.
In 2 biopsies, the radiolucent areas were composed of dense, collagenous fiber bundles with no signs of inflammation. These histologic features are in accordance with those of a dense, fibrous connective tissue,47 an “apical scar,”16 or a “periapical scar.”48 In one study,16 scar tissue healing was observed at the apices of teeth that had previously undergone periapical curettage; it was concluded that a periapical scar develops only after periapical surgery. However, in another histopathologic study,48 2.5% of 2308 specimens were diagnosed as periapical scars, most of which occurred at the apices of conventionally root-filled teeth. In our study, the observation of scar tissue in 2 specimens indicated that after root canal treatment the periapical lesion healed with soft connective tissue; during periapical surgery, most of the fibrous connective tissue might have been removed, allowing bone regeneration of the periapex to occur, as evidenced by the rapid postsurgical osseous healing (Fig 5).
Little is known about the tissue dynamics of periapical healing after conventional root canal treatment and periapical surgery; however, some deductions can be made from the data available on normal healing and guided regeneration of the marginal periodontium. Various tissue cells participate in the healing process. The pattern of healing depends on several factors, 2 of which are decisive: the regenerative potential49-51 and the speed with which the tissue cells bordering the defect react.52 A periapical scar probably develops because precursors of soft connective tissue colonize both the root tip and the periapical tissue; this may occur before the appropriate cells, which have the potential to restore the various structural components of the apical periodontium, are able to do so.
Our report confirms that persistent infection in the apical root canal system and cystic conditions of the periapical lesion are related to, and are probably important causes of, failed root canal treatment. Of these, only intraradicular infection can be directly affected by conventional re-treatment. True cysts and certain other potential etiologic factors, such as an extraradicular infection, foreign bodies, and cholesterol crystals, are situated outside the root canal system; such factors are less likely to be resolved by conservative endodontic treatment than by periapical surgery. The fact that the periapical area in every surgically treated case showed distinct bone healing (Table I and Figs 1 and 5) supports this contention. Interestingly, healing of the periapical lesion may occasionally occur by means of fibrous scar tissue, which appears as a periapical radiolucency indistinguishable from that seen in failed endodontic treatment. Surgical treatment may thus, in some instances, involve removal of a healed periapical scar.
We thank Mrs Susy Münzel-Pedrazzoli for competent technical assistance and Mrs Lilian Palmqvist and Sonia Andersson for excellent clinical assistance.
REFERENCES
1. Kakehashi S, Stanley HR, Fitzgerald RJ. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol 1965;20:340-9.
2. Möller ÅJR. Microbiological examination of root canals and periapical tissues of human teeth [thesis]. Göteborg, Sweden: Akademiförlaget, University of Göteborg; 1966.
3. Sundqvist G. Bacteriological studies of necrotic dental pulps [Dr Odont thesis]. Umeä, Sweden: University of Umeä; 1976.
4. Nair PNR. Light and electron microscopic studies of root canal flora and periapical lesions. Journal of Endodontics 1987;13:29-39.
5. Strindberg LZ. The dependence of the results of pulp therapy on certain factors: an analytic study based on radiographic and clinical follow-up examinations. Acta Odontol Scand 1956;14(Suppl 21):1-175.
6. Grahnén H, Hansson L. The prognosis of pulp and root canal therapy: a clinical and radiographic follow-up examination. Odontol Revy 1961;12:146-65.
7. Seltzer S, Bender IB, Turkenkopf S. Factors affecting successful repair after root canal treatment. J Am Dent Assoc 1963;67:651-62.
8. Storms JL. Factors that influence the success of endodontic treatment. J Can Dent Assoc 1969;35:83-97.
9. Molven O. The frequency, technical standard, and results of endodontic therapy. Norske Tannlaegeforenings Tidende 1976;86:142-7.
10. Kerekes K, Tronstad L. Long-term results of endodontic treatment performed with standardized technique. Journal of Endodontics 1979;5:83-90.
11. Molven O, Halse A. Success rates for gutta-percha and Klorperka N-Ø root fillings made by undergraduate students: radiographic findings after 10-17 years. International Endodontic Journal 1988;21:243-50.
12. Sjögren U, Hägglund B, Sundqvist G, Wing K. Factors affecting the long-term results of endodontic treatment. Journal of Endodontics 1990;16:498-504.
13. Sjögren U, Figdor D, Persson S, Sundqvist G. Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis. International Endodontic Journal 1997;30:297-306.
14. Sundqvist G, Figdor D, Persson S, Sjögren U. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1998;85:86-93.
15. Sundqvist G, Figdor D. Endodontic treatment of apical periodontitis. In: Ørstavik D, Pitt Ford TR, editors. Essential endodontology. Oxford: Blackwell; 1998. p. 242-77.
16. Seltzer S, Bender IB, Smith J, Freedman I, Nazimov H. Endodontic failures: an analysis based on clinical, roentgenographic, and histologic findings. Oral Surg Oral Med Oral Pathol 1967;23:500-30.
17. Andreasen JO, Rud J. A histobacteriologic study of dental and periapical structures after endodontic surgery. Int J Oral Surg 1972;1:272-81.
18. Block RM, Bushell A, Rodrigues H, Langeland K. A histopathologic, histobacteriologic, and radiographic study of periapical endodontic surgical specimens. Oral Surg Oral Med Oral Pathol 1976;42:656-78.
19. Langeland MA, Block RM, Grossman LI. A histopathologic and histobacteriologic study of 35 periapical endodontic surgical specimens. Journal of Endodontics 1977;3:8-23.
20. Lin LM, Pascon EA, Skribner J, Gängler P, Langeland K. Clinical, radiographic, and histologic study of endodontic treatment failures. Oral Surg Oral Med Oral Pathol 1991;71:603-11.
21. Nair PNR, Sjögren U, Kahnberg KE, Krey G, Sundqvist G. Intraradicular bacteria and fungi in root-filled, asymptomatic human teeth with therapy-resistant periapical lesions: a long-term light and electron microscopic follow-up study. Journal of Endodontics 1990;16:580-8.
22. Sjögren U, Happonen RP, Kahnberg KE, Sundqvist G. Survival of Arachnia propionica in periapical tissue. International Endodontic Journal 1988;21:277-82.
23. Nair PNR, Sjögren U, Krey G, Sundqvist G. Therapy-resistant foreign-body giant cell granuloma at the periapex of a root-filled human tooth. Journal of Endodontics 1990;16:589-95.
24. Simon JHS, Chimenti Z, Mintz G. Clinical significance of the pulse granuloma. Journal of Endodontics 1982;8:116-9.
25. Yusuf H. The significance of the presence of foreign material periapically as a cause of failure of root treatment. Oral Surg Oral Med Oral Pathol 1982;54:566-74.
26. Koppang HS, Koppang R, Solheim T, Aarneals H, Stølen SØ. Cellulose fibers from endodontic paper points as an etiologic factor in postendodontic periapical granulomas and cysts. Journal of Endodontics 1989;15:369-72.
27. Sedgley CM, Messer H. Long-term retention of a paper-point in the periapical tissues: a case report. Endodontics and Dental Traumatology 1993;9:120-3.
28. Nair PNR, Sjögren U, Schumacher E, Sundqvist G. Radicular cyst affecting a root-filled human tooth: a long-term post-treatment follow-up. International Endodontic Journal 1993;26:225-33.
29. Nair PNR, Sjögren U, Sundqvist G. Cholesterol crystals as an etiological factor in non-resolving chronic inflammation: an experimental study in guinea pigs. Eur J Oral Sci 1998;106:644-50.
30. Karnovsky MJ. A formaldehyde glutaraldehyde fixative of high osmolarity for use in electron microscopy. J Cell Biol 1965;27:137A-139A.
31. Schroeder HE, Rossinsky K, Müller W. An established routine method for differential staining of epoxy-embedded tissue sections. Microsc Acta 1980;83:111-6.
32. Fraska JM, Parks VR. A routine technique for double staining ultrathin sections using uranyl and lead salts. J Cell Biol 1965;25:157-61.
33. Venable JH, Coggeshall R. A simplified lead citrate stain for use in electron microscopy. J Cell Biol 1965;25:407-8.
34. Walton RE, Ardjmand K. Histological evaluation of the presence of bacteria in induced periapical lesions in monkeys. Journal of Endodontics 1992;18:216-21.
35. Sjögren U. Success and failure in endodontics [Dr Odont thesis]. Umeä, Sweden: Umeä University; 1996.
36. Nair PNR, Schroeder HE. Periapical actinomycosis. Journal of Endodontics 1984;10:567-70.
37. Figdor D, Sjögren U, Sorlin S, Sundqvist G, Nair PNR. Pathogenicity of Actinomyces israelii and Arachnia propionica: experimental infection in guinea pigs and phagocytosis and intracellular killing by human polymorphonuclear leukocytes in vitro. Oral Microbiol Immunol 1992;7:129-36.
38. Sundqvist G, Reuterving CO. Isolation of Actinomyces israelii from periapical lesion. Journal of Endodontics 1980;6:602-6.
39. Happonen RP. Periapical actinomycosis: a follow-up study of 16 surgically treated cases. Endodontics and Dental Traumatology 1986;2:205-9.
40. Byström A, Happonen RP, Sjögren U, Sundqvist G. Healing of periapical lesions of pulpless teeth after endodontic treatment with controlled asepsis. Endodontics and Dental Traumatology 1987;3:58-63.
41. O’Grady JF, Reade PC. Periapical actinomycosis involving Actinomyces israelii. Journal of Endodontics 1988;14:147-9.
42. Nair PNR, Pajarola G, Schroeder HE. Types and incidence of human periapical lesions obtained with extracted teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1996;81:93-102.
43. Nair PNR. Pathology of apical periodontitis, In: Pitt Ford TR, Ørstavik D, editors. Essential endodontology. London: Blackwell; 1998. p. 68-105.
44. Nair PNR. New perspectives on radicular cysts: do they heal? International Endodontic Journal 1998;31:155-60.
45. Simon JHS. Incidence of periapical cysts in relation to the root canal. Journal of Endodontics 1980;6:845-8.
46. Nair PNR. Apical periodontitis: a dynamic encounter between root canal infection and host response. Periodontology 2000 1997;13:121-48.
47. Penick E. Periapical repair by dense fibrous connective tissue following conservative endodontic therapy. Oral Surg Oral Med Oral Pathol 1961;14:239-42.
48. Bhaskar SN. Periapical lesion: types, incidence, and clinical features. Oral Surg Oral Med Oral Pathol 1966;21:657-71.
49. Karring T, Nyman S, Lindhe J. Healing following implantation of periodontitis affected roots into bone tissue. J Clin Periodontol 1980;7:96-105.
50. Nyman S, Gottlow J, Karring T, Lindhe J. The regenerative potential of the periodontal ligament: an experimental study in the monkey. J Clin Periodontol 1982;9:257-65.
51. Karring T, Nyman S, Gottlow J, Laurell L. Development of the biological concept of guided tissue regeneration: animal and human studies. Periodontology 2000 1993;1:26-35.
52. Schroeder HE. The periodontium. Vol. 5. In: Schroeder HE. Handbook of microscopic anatomy. Berlin: Springer-Verlag; 1986.
Reprint Requests:
P. N. R. Nair, BVSc, DVM, PhD
Department of Oral Structural Biology
Plattenstrasse 11
CH-8028 Zurich
Switzerland
aDepartment of Oral Structural Biology, Center of Dental and Oral Medicine, University of Zurich.
bDepartment of Endodontics, Umeå University.
cSchool of Dental Sciences, University of Melbourne.
Received for publication Oct 6, 1998; returned for revision Dec 12, 1998; accepted for publication Jan 12, 1999.
Copyright © 1999 by Mosby, Inc.
1079-2104/99/$8.00 + 0 7/15/97344