Vol. 92 No. 2 August 2001

| ENDODONTICS | Editor: Larz Spångberg |
A new bacterial species associated with failed endodontic treatment: Identification and description of Actinomyces radicidentis
Sotirios Kalfas, DDS, PhD,a David Figdor,
BDSc, MDSc, FRACDS, Dip Endo,b and Göran Sundqvist,
DDS, PhD,c Umeå, Sweden; Thessaloniki, Greece; and
Melbourne, Australia
UMEÅ UNIVERSITY, ARISTOTLE UNIVERSITY, AND UNIVERSITY OF MELBOURNE
Objective. This report describes 2 endodontic patients who had persistent signs and symptoms after conventional root canal treatment. The aim of this study was to determine what microorganisms were present in the root canals of the teeth with failed endodontic therapy.
Study design. After removal of the root fillings, the canals were sampled by advanced microbiological techniques and the isolates were characterized by various tests.
Results. Bacteria, which grew in pure cultures, were isolated in each case. The bacteria were similar to each other and were classified as Actinomyces on the basis of phylogenic and phenotypic evidence. The bacteria were different from others within the genus, thus warranting designation as a new species, Actinomyces radicidentis.
Conclusions. The 2 cases of endodontic failure were infected with
A radicidentis, a new Actinomyces species. This bacterium joins a restricted group of
other microorganisms that have been associated with failure of root canal treatment.
(Oral
Surg Oral Med Oral Pathol Oral Radiol Endod 2001;92:208-14)
When teeth are treated according to accepted clinical principles under aseptic conditions, the success rate of endodontic treatment is generally high—most follow-up studies report overall success rates of 85% to 90%.1-6
It is commonly acknowledged that most endodontic treatment failures occur when treatment procedures have not met a satisfactory standard for control and elimination of infection. However, even when endodontic treatment has been performed to a high standard, long-term follow-up has shown that some cases exhibit persistence or development of a periapical radiolucency. Factors that may contribute to the perpetuation of periapical radiolucencies after root canal treatment include the following: intraradicular infection persisting in the apical part of the root canal7; extraradicular infection, generally in the form of peri-apical actinomycosis8; extruded root canal filling or other materials that cause foreign body reactions9-13; and true cysts, especially those with a significant accumulation of cholesterol crystals.14,15 In some cases, a persisting periapical radiolucency may be mistaken for failure because of the formation of scar tissue.16
Information obtained from histologic analysis suggests that there is a ceiling to the success rate that can be achieved by conventional endodontic treatment of apical periodontitis.17 Assuming that periapical actinomycosis and true cysts do not heal by conventional root canal treatment, the total frequency of these 2 conditions will determine the highest possible healing rate that can be attained after optimal endodontic therapy. Successful elimination of the initial root canal infection and complete obturation of the canal will result in periapical healing unless the lesion happens to be a true cyst or one that is maintained by bacteria in the periapical tissue. Actinomyces species and Propionibacterium propionicum (formerly Arachnia propionica), which have been implicated in periapical infections, occur in 10% to 15% of the root canals of teeth with periapical lesions18,19; however, only a few of these teeth develop periapical actinomycosis. Relatively little is known about the incidence of periapical actinomycosis, although the limited available data suggest that it is probably less than 5% of all periapical lesions.20,21
Both Actinomyces israelii and P propionicum have been repeatedly found in the periapical tissues of cases that do not respond to conventional endodontic treatment.22–24 A israelii has been specifically implicated in periapical actinomycosis.22,24 Our report supplements previous accounts of bacteria associated with failure of conventional endodontic treatment. Two cases are described in which endodontic therapy failed and conventional retreatment had to be followed by periapical surgery to resolve clinical symptoms and ensure periapical healing. A new species of Actinomyces was isolated in pure cultures from the root canals of the involved teeth.
MATERIAL AND METHODS
Clinical material
The 2 cases reported here have been briefly described in a recent report on the taxonomic status of the isolated bacteria.25 The 2 strains were isolated from root canals of teeth in separate patients with similar clinical histories. A detailed description of the cases is outlined here.
In case MK, a 25-year-old man, strain CCUG 42377 (Culture Collection University Gothenburg, Sweden) was isolated in a sample taken from the root canal of an upper central incisor with apical periodontitis (Fig 1). Both the central and the lateral incisors had been devitalized after orthodontic treatment more than 10 years earlier. No periapical lesions were present at the commencement of endodontic treatment; however, there was evidence of apical root resorption. Because of persistent symptoms, the teeth were not root-filled for 7 years but were instead dressed with calcium hydroxide paste and sealed with temporary fillings. During this period, the temporary fillings were lost several times. The dentist treating the teeth also reported that there had been problems isolating the teeth with rubber dam during the initial treatment because of the orthodontic appliances. Eventually, periapical lesions developed and the patient was ultimately referred to an endodontist who completed the treatment and root-filled the teeth. Three years after the teeth were root-filled, a periapical abscess developed in the region. Penicillin was administered, and the root fillings were removed under aseptic conditions. The canals were instrumented with sodium hypochlorite (0.5%) irrigation, followed by dressing with a calcium hydroxide paste. A new abscess developed, so a decision was made to carry out periapical surgery. Before surgery, bacteriologic samples were taken and the root canals were filled.
In case GL, an 80-year-old woman, strain CCUG 36733 was isolated from the root canal of a previously obturated tooth. A dentist had treated the upper right canine on repeated occasions. Despite persistent symptoms, the tooth was root-filled. The dentist subsequently referred the patient to an endodontist for treatment. The patient reported that the previous treatment had been performed without rubber dam isolation of the tooth. The root filling was removed and a bacteriologic sample taken. The canal was instrumented and irrigated with sodium hypochlorite solution (0.5%), then dressed with a calcium hydroxide paste. The strain in the initial sample grew as a pure culture. At 3 subsequent treatments, bacteriologic samples were obtained and all showed growth of the same type of bacterium. Because of continuing symptoms, periapical surgery was performed on the tooth and granulomatous tissue was removed. The surgical postoperative healing was uneventful.
Bacteriologic sampling
An aseptic technique, described by Möller,26 was used during all specialist treatment and bacteriologic sampling. Rubber dam was applied, and the tooth, clamp, and surrounding parts of the rubber dam were cleaned with 30% hydrogen peroxide and then swabbed with 5% iodine tincture. After the tincture had dried, the tooth surface was swabbed with 5% sodium thiosulphate solution to inactivate the iodine so that remnants of iodine would not influence the bacteriologic sampling.26 Sterile saline solution was carefully introduced into the canal with a syringe, taking care that the root canal would not be overfilled. The canal was instrumented so that material could be obtained from the dentin walls. The fluid in the root canal was absorbed with charcoaled paper points, which were transferred to a liquid thioglycolate medium (11260; Baltimore Biological Laboratories, Cockeysville, Md) supplemented with agar to prevent oxygen diffusion.27 This medium is highly effective in reducing oxygen so that toxic intermediates of oxygen do not accumulate even if the medium is briefly exposed to oxygen.28
Microbiological examination of the samples
The samples were transferred to an anaerobic box, where the paper points were placed in 1-mL prereduced buffer solution and shaken vigorously to release the adsorbed microbes. The suspension was inoculated on Brucella agar (Becton, Dickinson and Company, Franklin Lakes, NJ) supplemented with 5% lysed blood, 0.05 mg/mL hemin, and 0.01 mg/mL vitamin K. The plates were incubated anaerobically (10% H2 and 5% CO2 in nitrogen) at 36°C for up to 10 days. Bacteria grown on the plates were isolated and characterized using routine identification tests,29,30 including growth on selective media and gas-liquid chromatography for detection of metabolic end products in prereduced anaerobically sterilized broth cultures.31
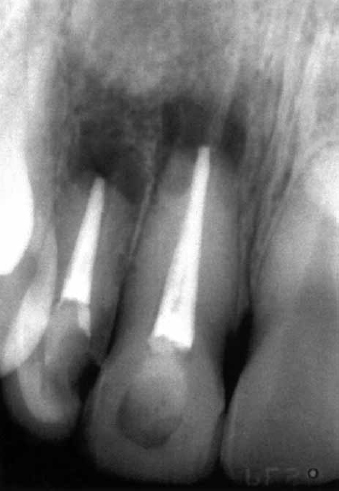
Fig 1. Periapical radiograph of upper right central and lateral incisors of case MK when periapical abscess developed 3 years after root filling. Bacteriologic sample was taken from root canal of upper central incisor during retreatment, which revealed presence of A radicidentis strain CCUG 42377.
Electron microscopy
Samples were prepared for electron microscopy by growing pure cultures of the bacterial isolates on Brucella blood agar (3% laked horse blood) or MS agar, in Tryptic soy broth with 0.5% glucose and 2% horse serum, or in RPMI-1640 broth (Sigma-Aldrich, St Louis, Mo) with or without 10% calf serum. Cells were harvested, washed twice with phosphate-buffered saline (PBS), and fixed with 2.5% glutaraldehyde in PBS. Samples were prepared for electron microscopy according to routine procedures.
Susceptibility to sodium hypochlorite and calcium hydroxide
A radicidentis was tested for its susceptibility to sodium hypochlorite and calcium hydroxide using a method described previously.32 Briefly, each strain of A radicidentis was grown anaerobically (10% CO2 and 10% H2 in nitrogen) on brain heart infusion agar (Oxoid; Basingstroke, United Kingdom) and incubated at 37°C for 7 to 14 days. Colonies were harvested, suspended in 1-mL PBS, ground with a glass pestle, and vortexed to produce a fine cell suspension.
Aliquots (100 µL) of this suspension were placed in 1 mL of either 0.5% sodium hypochlorite solution or a saturated solution of calcium hydroxide (pH 11.5) and incubated aerobically at 21°C for 5, 15, and 30 minutes. Controls were incubated in PBS for 30 minutes. After incubation, the cells were pelleted, washed twice in PBS, serially diluted in 0.9% NaCl, and plated onto brain heart infusion agar; viable counts were determined after incubation for 1 week. Experiments were repeated in duplicate.
RESULTS
In both cases—in the central incisor of case MK and in the upper right canine of case GL—the teeth had previously been root-filled and the samples were obtained after removal of these root fillings. The bacterium isolated from the root canal of each patient (strains CCUG 42377 and CCUG 36733, respectively) grew in pure cultures. In case MK, the central incisor was sampled before periapical surgery and no other samples were taken. In case GL, the right canine was treated on several occasions by instrumentation and irrigation with 0.5% sodium hypochlorite solution, followed by interappointment dressing with a calcium hydroxide paste (Calasept; Nordiska Dental, Ängelholm, Sweden). At 3 appointments, samples taken from this tooth showed growth of the same microorganism in pure culture. Follow-up of both cases showed healing of the periapical tissues.
The 2 bacterial isolates did not conform to other known species within the genus Actinomyces. The results of whole-cell protein analysis and 16S rRNA gene sequencing showed that the strains closely resembled each other but were sufficiently distinct from other Actinomyces species to be deemed a new species within the genus. The name Actinomyces radicidentis was given to the new species.25 In addition to the biochemical characteristics described in the first report,25 the isolates were found to produce acetic, lactic, and succinic acids in glucose-containing prereduced anaerobically sterilized broth. Under aerobic conditions (with 5% CO2), the isolates grew within 2 days on Mitis Salivarius agar (Difco, Detroit, Mich) but failed to grow on 110 Staphylococcus agar (Difco), Rogosa selective lactobacilli agar (Difco), or bile esculin agar29 for enterococci. On Brucella blood agar, the growth was butyrous and colonies easily formed a smooth suspension when mixed with buffer. The broth cultures were difficult to suspend homogeneously.
Cells from agar or broth cultures stained Gram positive and exhibited a coccoid cell shape, with the cells arranged in irregular clusters resembling a bunch of grapes, and were indistinguishable from staphylococci or some micrococci. Scanning electron microscopy of the isolates grown on Brucella blood agar (Fig 2, A) or in serum-supplemented Tryptic soy broth (Fig 2, B) revealed a coccoid cell shape, although cells grown in RPMI-1640 broth with serum were rod-shaped (Fig 2 C, D). When cells were grown in broth, scanning electron microscopy revealed the existence of bundles of thick fimbriae-like appendices in a net arrangement (Fig 2, B-D). The presence of fimbriae was confirmed in thin sections (Fig 2, E-G).
Because A radicidentis was recovered from canals after repeated irrigation with sodium hypochlorite solution and dressing with calcium hydroxide, we examined survival of A radicidentis in these solutions in vitro. Both A radicidentis strains were killed efficiently by sodium hypochlorite solution—there was no growth at or after 5 minutes. The average survival of A radicidentis strains CCUG 42377 and CCUG 36733 in calcium hydroxide after 5, 15, and 30 minutes was 14, 0.1, and 1 × 10–4% and 28, 5.8, and 9 × 10–2%, respectively.
DISCUSSION
Identification of the biological reasons for endodontic failure is fundamental for progress to be made in understanding the microbial and nonmicrobial pathogenesis of such failure. This article describes the clinical characteristics and some in vitro properties of a new Actinomyces species isolated from 2 independent cases of endodontic failure. In both patients, the protracted course of previous clinical treatment led the endodontist to suspect that the continuing signs and symptoms were due to a persistent infection. Thus, bacteriologic samples were taken and sent to the microbiological laboratory with the suspicion that they might be derived from teeth with periapical actinomycosis. On 3 separate occasions, the samples from the canine (case GL) consistently showed growth of the same microorganism, A radicidentis. In the other case (MK), the specialist treated the upper incisors in 2 appointments; the bacteriologic samples were taken at the second, before root filling and apical surgery. Both incisors harbored the same species, A radicidentis.
Because this is the first description of this Actinomyces species, it is not known whether it is a part of the initial flora of infected root canals, nor is the natural habitat of the species identified. The mode of entry into the canals by A radicidentis is also uncertain—it might have been present in the initial root canal infection; alternatively, A radicidentis may have entered the root canal during a phase of treatment in which the asepsis was deficient and it might have used an opportunity to survive when other microbes were removed during endodontic treatment. A common feature in both cases was that the initial treatment of the canals was performed in the absence of an acceptable aseptic technique. Poor (or no) isolation with rubber dam, no disinfection of the operative field, and lack of an adequate interappointment seal of the access cavity can facilitate entry of opportunistic bacteria into the root canal. It has recently been reported that opportunistic microorganisms such as Enterococcus faecalis and Candida albicans are detectable in a higher proportion of cases in which the treatment has been protracted and the asepsis has been poor during treatment.33,34 Discovery of the natural habitat of A radicidentis should provide a better understanding of how these bacteria can enter and survive in the root canal.
The persistence of A radicidentis in both cases and the repeated recovery of the species on 3 separate occasions from one case (GL) imply that there was a nidus of infection, either intraradicularly or extraradicularly. Because no immunocytochemical or electron microscopic analysis was performed on the periapical tissue, we are unable to specify where the bacteria were located. A radicidentis may have survived extraradicularly in the form of periapical actinomycosis, or the species might have the capacity to persist within the root canal system. In both patients, the canals were irrigated with sodium hypochlorite solution during instrumentation and dressed with calcium hydroxide, yet A radicidentis was repeatedly recovered from the canals. Thus, we examined survival of A radicidentis in these antimicrobial agents in vitro. Both strains were killed rapidly by 0.5% sodium hypochlorite solution. The strains exhibited relatively high tolerance to saturated calcium hydroxide solution in comparison with that of other bacteria isolated from infected root canals.35 The persistence of A radicidentis might be due to an extraradicular location of A radicidentis in the periapical tissues, but there is also the possibility that the calcium hydroxide dressing was ineffective at eliminating A radicidentis or that the bacteria were inaccessible by the medicaments used during treatment of the root canal system.
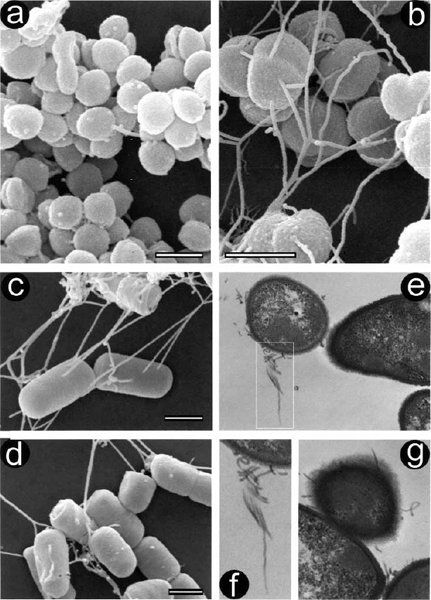
Fig 2. Scanning (a-d) and transmission electron micrographs (e-g) of A radicidentis, strain CCUG 36733. Cells grown on Brucella blood agar exhibit coccoid shape (a), similar to cells grown in serum-supplemented Tryptic soy broth, where fimbriae-like bundles can be seen (b). When cells were grown in RPMI-1640 broth with serum (c, d), they were rod-shaped with intertwining bundles of fimbriae-like cell appendices in a netlike arrangement. In thin section (e), bundle of fimbriae can be observed emerging from cell surface; demarcated area in (e) is magnified in (f). Further thin section (g) shows radially placed fimbriae emerging from fuzzy-coated cell surface. Bars = 1 µm.
Periapical actinomycosis is generally thought to be associated with infection by A israelii, and the clinical course of persistent infection, problematic treatment, and endodontic failure22-24 bears considerable similarity to the cases described here. The ability of A israelii to evade destruction and elimination by host phagocytic cells by establishing characteristic cohesive colonies consisting of branching filamentous organisms in the periapical tissue seems to be the key to their pathogenicity.36 The A radicidentis strains CCUG 36733 and 42377 did not grow as branching filaments in vitro, although a change to a rodlike morphology occurred when A radicidentis was grown in serum-supplemented RPMI-1640 broth. The presence of thick, ropelike cell structures in a net arrangement observed on broth-grown A radicidentis is characteristic of fimbriae; this was confirmed by means of electron microscopy of thin sections. Fimbriae have been reported on some strains of A israelii37 and other Actinomyces species.38,39 These structures are thought to be important for adhesion to host tissue and other bacterial surfaces.40
Microscopically, the cells of A radicidentis were predominantly coccoid; this morphology misled us so that the isolates were initially not recognized as Actinomyces species. It is well known that growth conditions can influence cell morphology, as observed here with A radicidentis, which appeared rodlike when grown in RPMI-1640 broth with serum. Another species involved in endodontic failure, P propionicum, has been reported to grow with branching filaments or with swollen spherical cells—or occasionally it may consist entirely of coccoid forms.41 It is possible that the strains isolated from these cases in vivo can also form filaments; a branching filamentous growth seems important for establishment of the bacteria in host tissue.36,42,43
When using routine procedures to identify A radicidentis in clinical samples, the close resemblance of A radicidentis to staphylococci (in cell shape and arrangement, colony morphology, and production of catalase) can complicate classification of the species. Nevertheless, the biochemical profile of A radicidentis differs from the most clinically significant Staphylococcus species when such a profile is based on key characteristics.44 The most suitable differentiation criterion at the genus level is the production of succinic acid, together with acetic and lactic acids, from cultures in glucose-containing media. Further differentiation from other catalase-producing Actinomyces species can be made with the tests described earlier25 or by comparing 16S rRNA data with sequence data deposited at Genbank under accession number AJ251986. The classification of A radicidentis as a distinct species is an addition to the growing number of species within the genus Actinomyces.
Our ability to define appropriate treatment strategies for problematic cases can only follow identification and characterization of the etiologic factors that result in endodontic treatment failure. The cases reported here—along with the identification of a new species, A radicidentis—supplement our knowledge of a small, restricted group of microorganisms known to be associated with endodontic failure.
REFERENCES
1. Strindberg LZ. The dependence of the results of pulp therapy on certain factors: an analytic study based on radiographs and clinical follow-up examinations. Acta Odontol Scand 1956; 14(Suppl 21):1-175.
2. Grahnén H, Hansson L. The prognosis of pulp and root canal therapy: a clinical and radiographic follow-up examination. Odontol Revy 1961;12:146-65.
3. Seltzer S, Bender IB, Turkenkopf S. Factors affecting successful repair after root canal therapy. J Am Dent Assoc 1963;67:651-62.
4. Kerekes K, Tronstad L. Long-term results of endodontic treatment performed with a standardized technique. J Endod 1979;5:83-90.
5. Molven O, Halse A. Success rates for gutta-percha and Kloroperka N-Ø root fillings made by undergraduate students: radiographic findings after 10-17 years. Int Endod J 1988;21:243-50.
6. Sjögren U, Hägglund B, Sundqvist G, Wing K. Factors affecting the long-term results of endodontic treatment. J Endod 1990;16:498-504.
7. Nair PNR, Sjögren U, Krey G, Kahnberg KE, Sundqvist G. Intraradicular bacteria and fungi in root-filled, asymptomatic human teeth with therapy-resistant periapical lesions: a long-term light and electron microscopic follow-up study. J Endod 1990;16:580-8.
8. Sjögren U, Happonen RP, Kahnberg KE, Sundqvist G. Survival of Arachnia propionica in periapical tissue. Int Endod J 1988;21:277-82.
9. Simon JH, Chimenti RA, Mintz GA. Clinical significance of the pulse granuloma. J Endod 1982;8:116-9.
10. Yusuf H. The significance of the presence of foreign material periapically as a cause of failure of root treatment. Oral Surg Oral Med Oral Pathol 1982;54:566-74.
11. Koppang HS, Koppang R, Solheim T, Aarnes H, Stølen SØ. Cellulose fibers from endodontic paper points as an etiological factor in postendodontic periapical granulomas and cysts. J Endod 1989;15:369-72.
12. Nair PNR, Sjögren U, Krey G, Sundqvist G. Therapy-resistant foreign body giant cell granuloma at the periapex of a root-filled human tooth. J Endod 1990;16:589-95.
13. Sedgley CM, Messer H. Long-term retention of a paper point in the periapical tissues: a case report. Endod Dent Traumatol 1993;9:120-3.
14. Nair PNR, Sjögren U, Schumacher E, Sundqvist G. Radicular cyst affecting a root-filled human tooth: a long-term post-treatment follow-up. Int Endod J 1993;26:225-33.
15. Nair PNR. New perspectives on radicular cysts: do they heal? Int Endod J 1998;31:155-60.
16. Nair PNR, Sjögren U, Figdor D, Sundqvist G. Persistent periapical radiolucencies of root-filled human teeth, failed endodontic treatments, and periapical scars. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999;87:617-27.
17. Sjögren U. Success and failure in endodontics. Umeå, Sweden: Umeå University; 1996. Odontological Dissertation No 60.
18. Borssén E, Sundqvist G. Actinomyces of infected dental root canals. Oral Surg Oral Med Oral Pathol 1981;51:643-8.
19. Sundqvist G. Taxonomy, ecology, and pathogenicity of the root canal flora. Oral Surg Oral Med Oral Pathol 1994;78:522-30.
20. Nair PNR, Schroeder HE. Periapical actinomycosis. J Endod 1984;10:567-70.
21. Byström A, Happonen RP, Sjögren U, Sundqvist G. Healing of periapical lesions of pulpless teeth after endodontic treatment with controlled asepsis. Endod Dent Traumatol 1987;3:58-63.
22. Sundqvist G, Reuterving CO. Isolation of Actinomyces israelii from periapical lesion. J Endod 1980;6:602-6.
23. Happonen R-P. Periapical actinomycosis: a follow-up study of 16 surgically treated cases. Endod Dent Traumatol 1986;2:205-9.
24. O’Grady JF, Reade PC. Periapical actinomycosis involving Actinomyces israelii. J Endod 1988;14:147-9.
25. Collins MD, Hoyles L, Kalfas S, Sundqvist G, Monsen T, Nikolaitchouk N, et al. Characterization of Actinomyces isolates from infected root canals of teeth: description of Actinomyces radicidentis sp. nov. J Clin Microbiol 2000;38:3399-3403.
26. Möller ÅJR. Microbiological examination of root canals and periapical tissues of human teeth. Methodological studies. Odontol Tidskr 1966;74:(Suppl):1-380.
27. Carlsson J, Sundqvist G. Evaluation of methods of transportation and cultivation of bacterial specimens from infected root canals. Oral Surg Oral Med Oral Pathol 1980;49:451-4.
28. Carlsson J, Nyberg G, Wrethén J. Hydrogen peroxide and super-oxide radical formation in anaerobic broth media exposed to atmospheric oxygen. Appl Environ Microbiol 1978;36:223-9.
29. Baron EJ, Peterson LR, Finegold SM, editors. Bailey & Scott’s diagnostic microbiology. 9th ed. St. Louis: Mosby–Year Book; 1994.
30. Koneman EW, Allen SD, Janda WM, Schreckenberger PC, Winn WC Jr, editors. Color atlas and textbook of diagnostic microbiology, 5th ed. Philadelphia: Lippincott-Raven Publishers; 1997.
31. Holdeman LV, Cato EP, Moore WE. Anaerobe laboratory manual. 4th ed. Blacksburg, VA: Virginia Polytechnic Institute Anaerobe Laboratory; 1977.
32. Barnard D, Davies J, Figdor D. Susceptibility of Actinomyces israelii to antibiotics, sodium hypochlorite and calcium hydroxide. Int Endod J 1996;29:320-6.
33. Siren EK, Haapasalo MPP, Ranta K, Salmi P, Kerosuo ENJ. Microbiological findings and clinical treatment procedures in endodontic cases selected for microbiological investigation. International Endodontic Journal 1997;30:91-5.
34. Waltimo TMT, Siren EK, Torkko HLK, Olsen I, Haapasalo MPP. Fungi in therapy-resistant apical periodontitis. International Endodontic Journal 1997;30:96-101.
35. Byström A, Claesson R, Sundqvist G. The antibacterial effect of camphorated paramonochlorophenol, camphorated phenol and calcium hydroxide in the treatment of infected root canals. Endod Dent Traumatol 1985;1:170-5.
36. Figdor D, Sjögren U, Sörlin S, Sundqvist G, Nair PNR. Pathogenicity of Actinomyces israelii and Arachnia propionica: experimental infection in guinea pigs and phagocytosis and intracellular killing by human polymorphonuclear leukocytes in vitro. Oral Microbiol Immunol 1992;7:129-36.
37. Figdor D, Davies J. Cell surface structures of Actinomyces israelii. Aust Dent J 1997;42:125-8.
38. Lai C-H, Listgarten MA. Immune labeling of certain strains of Actinomyces naeslundii and Actinomyces viscosus by fluorescence and electron microscopy. Infect Immun 1979;25:1016-28.
39. Cisar JO. Fimbrial lectins of the oral Actinomyces. In: Mirelman D, editor. Microbial lectins and agglutinins. New York: Wiley; 1986. p. 183-96.
40. Klemm P. Fimbriae. Adhesion, genetics, biogenesis, and vaccines. Boca Raton (FL): CRC Press; 1994.
41. Slack JM, Gerencser MA, editors. Actinomyces, filamentous bacteria. Biology and pathogenicity. Minneapolis: Burgess Publishing; 1975.
42. Brown JR, von Lichtenberg F. Experimental actinomycosis in mice. Study of pathogenesis. Arch Pathol 1970;90:391-402.
43. Behbehani MJ, Jordan HV. Comparative pathogenicity of Actinomyces species in mice. J Med Microbiol 1982;15:465-73.
44. Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH, editors. Manual of clinical microbiology. 7th ed. American Society for Microbiology, Washington, DC; 1999. p. 264-82.
Reprint requests:
G. Sundqvist
Department of
Endodontics
School of Dentistry
Umeå University, SE-901 87
Umeå,
Sweden
[email protected]
AVAILABILITY OF JOURNAL BACK ISSUES
As a service to our subscribers, copies of back issues of Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics for the preceding 5 years are maintained and are available for purchase from the publisher, Mosby, until inventory is depleted. Please write to Mosby, Subscription Customer Service, 6277 Sea Harbor Dr, Orlando, FL 32887, or call 800-654-2452 or 407-345-4000 for information on availability of particular issues and prices. If unavailable from the publisher, photocopies of complete issues are available from Bell & Howell Information and Learning, 300 N Zeeb Rd, Ann Arbor, MI 48106-1346; (734) 761-4700 or (800) 521-0600.
aAssociate Professor, Department of Oral Microbiology, Umeå University, Umeå, Sweden; Department of Preventive Dentristry and Periodontology, Aristotle University, Thessaloniki, Greece.
bSenior Fellow, School of Dental Science, University of Melbourne, Australia.
cProfessor, Department of Endodontics, Umeå University, Umeå, Sweden. Received for publication Apr 10, 2001; returned for revision Apr 15, 2001; accepted for publication May 2, 2001.
Copyright © 2001 by Mosby, Inc.
1079-2104/2001/$35.00 + 0 7/15/117268
doi:10.1067/moe.2001.117268