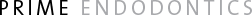| Oral Microbiology Immunology 2003: 18: 234–239 | Copyright © Blackwell Munksgaard 2003 |
| Printed in Denmark. All rights reserved | Qral MicrobioIogy and lmmunology |
| ISSN 0902-0055 |
Starvation survival, growth and recovery of Enterococcus faecalis in human serum
D. Figdor1,2, J. K. Davies1, G. Sundqvist2
1Department of Microbiology, Monash University, Melbourne, Australia,
2Department of Endodontics, Umeå University, Umeå, Sweden
Figdor D, Davies JK, Sundqvist G. Starvation survival, growth and recovery of Enterococcus faecalis in human serum.
Oral Microbiol Immunol 2003: 18: 234–239. © Blackwell Munksgaard, 2003.
The ability of Enterococcus faecalis to survive starvation for long periods in the obturated root canal is likely to be an important factor in the pathogenesis and maintenance of a persistent infection after endodontic treatment. The response of E. faecalis to starvation survival in water and glucose-, phosphate- or amino acid-limited chemically defined medium was studied, along with the capacity for growth and recovery of starved cells of E. faecalis in pooled human serum. After an initial rapid fall in cell numbers, a small remaining population of E. faecalis was able to survive in water for over 4 months and in nutrient-limited media for extended periods. A high cell density at the onset of starvation was critical for the ability of E. faecalis to endure prolonged nutrient limitation. Upon starvation, a static population of starved cells developed and were apparently in a minimal metabolic state, since blocking cell wall synthesis with penicillin G or inhibiting DNA synthesis with norfloxacin during starvation resulted in limited change in the rate of loss of viable cells. In 50% serum, E. faecalis grew, then stabilized at a relatively constant population of 106 colony-forming units/ml for 4 months, irrespective of the initial cell density. In summary, E. faecalis is capable of withstanding prolonged periods of starvation in a minimal metabolic state provided that there is a high cell density at the onset of starvation. Starved cells were capable of recovery upon addition of human serum.
Key words: Enterococcus faecalis; starvation; serum
Dr David Figdor, 517 St Kilda Road,
Melbourne, VIC 3004 Australia
Tel.: +61 3 9866 4528; fax: +61 3 9820 3102;
e-mail: [email protected]
Accepted
for publication January 9, 2003
The principal cause of root canal treatment failure is generally thought to be the persistence of microorganisms in the apical part of root filled teeth (39, 40). The microbial flora in canals after failed endodontic therapy is unlike that found in untreated teeth, as only one or a few microbial species are present, there is a smaller proportion of anaerobic bacteria, and Gram-positive bacteria dominate the infection (19, 33, 34, 49). Enterococci are the most frequently isolated bacteria from the canals of root filled teeth with persistent periapical lesions (19, 33, 34, 44, 45, 49).
Enterococcus faecalis is the most prevalent species of enterococci in root canals of failed cases (45, 49) and for it to be involved in the pathogenesis and maintenance of apical periodontitis, it must survive in the root filled canal where the nutrient supply is limited. E. faecalis has been shown to have a relatively uncommon capacity to survive in root canals as a monoculture without the support of other bacteria (12). E. faecalis is also known to survive periods of starvation in water (20) and in infected dentine in water for periods of at least 10 days (43). A capacity to endure starvation is a distinctive characteristic that might allow E. faecalis to survive until an opportunity for acquiring suitable nutrition becomes available. For example, after a period of time, breakdown of the endodontic sealer and microleakage around a root filling may allow tissue fluid to enter the root canal space and induce recovery of starved cells.
Remarkably little is known about what nutritional substrate is available for microorganisms in the root filled canal. Information from ultrastructural investigations of failure cases suggests that bacteria that survive obturation are likely to be located either in voids adjacent to dentine, or within dentine in apical areas of the root canal adjacent to a periapical granuloma (40). The presence of microorganisms in these locations suggests that the nutrition required to sustain the microbial flora probably comes from fluid in the periapical tissues. Although the fluid has not been characterized, it is likely to be composed of a serum-like substance derived from surrounding bone and connective tissue and capable of supporting growth of selected microorganisms (28, 35).
The starvation-survival response has been studied in many Gram-negative bacteria, especially Vibrio cholerae and Escherichia coli (26, 50); however, only a few studies have examined starvation in Gram-positive bacteria, in particular Micrococcus luteus (38) and Staphylococcus aureus (51). Several studies have shown that E. faecalis can withstand periods of starvation in low-glucose media or water (15, 16, 20) and that synthesis of stress-induced proteins is important for survival. The dynamics of the starvation response and role of environmental and cell-density related factors has not been evaluated in E. faecalis, yet an ability to endure periods of limited nutrition or starvation is likely to be an important characteristic in the pathogenesis of persistent apical periodontitis.
The aims of this study were to establish the starvation-survival kinetics of E. faecalis, to characterize several factors that affect survival of starved cells during prolonged incubation, and to determine the ability of E. faecalis to recover from starvation in the presence of human serum.
Materials and methods
Bacterial strains and starvation media
Enterococcus faecalis strain JH2-2, which is derived from the parental strain JH2 (22) and representative of the species, was used in all experiments. Two other E. faecalis strains, OMGS 3264 and OMGS 3372, originally isolated from dental root canals (8) and obtained from the Department of Oral Microbiology, Göteborg University, Sweden, were used in some experiments. Bacteria were grown in a chemically defined medium (CDM) (21).
Glucose-limited and phosphate-limited media were prepared by lowering the glucose concentration from 1% (wt/vol.) to 0.1% and the phosphate concentration from 0.18 M to 36 nM of CDM. An amino acid-limited CDM was prepared by deleting the amino acids from the CDM formula (21). Distilled, filtered sterile water was used in prolonged starvation experiments.
Growth and starvation of cells
Cells were grown by inoculating E. faecalis in CDM, incubating at 37°C with shaking, in air, for 24 h. After overnight culture, cells were harvested by centrifugation (3200 ×g for l0 min), washed twice in phosphate-buffered saline (PBS) by repeated centrifugation and resuspended in PBS. Starvation cultures were prepared by inoculating the final suspension at approximately 108 colony-forming units (cfu/ml) in various nutrient-limited media, including phosphate- and glucose-limited CDM, amino-acid-free CDM, PBS, and water, incubated at 37°C. Starvation-survival kinetics of E. faecalis were established over a 21-day period, but some prolonged starvation cultures extended for over 4 months. The role of cell density in starvation survival was determined in experiments with starting cell densities of approximately 104 to 108 cfu/ml.
Starvation-survival was determined by viable counts of serial dilutions in PBS; plating on CDM agar, and incubation at 37°C for 48 h. All incubation was in air, except for experiments evaluating the role of anaerobiosis on starvation survival where E. faecalis was incubated anaerobically (10% H2 and 5% CO2 in nitrogen) at 37°C. For anaerobic experiments, PBS and distilled water were pre-reduced for at least 24 h in an anaerobic box.
Growth, survival and recovery of starved cells in human serum
Sera from three healthy human adults were pooled, inactivated at 56°C for 30 min, and stored at –80°C until used. Growth in pooled human serum (PHS) was determined by inoculation of mid-log phase cells into PHS (1%, 5%, and 50% PHS, diluted in PBS). Long-term survival of E. faecalis strain JH2-2 in serum was determined by inoculating cells at densities of 101–104 cfu/ml in 50% PHS, with stationary incubation at 37°C for more than 4 months. Viable counts were determined by serial dilution in PBS and plating on CDM agar.
Seven-day starved cells were recovered by mixing serum with the starvation cultures to give a final serum concentration of 50%. After serum addition, the cultures were incubated at 37°C during the recovery period. Viable counts were determined as above.
Cell metabolism during starvation
Starvation cultures were established in water and glucose-limited CDM. At 7d after onset of starvation, the cell-wall-synthesis inhibitor penicillin G, or the DNA synthesis inhibitor norfloxacin, was added to starvation cultures. Penicillin G was added to give a final concentration of 20µg/ml (7 times the MIC), and norfloxacin at 30µg/ml (10 times the MIC). Viable counts were established as for starvation survival kinetics.
Results
Starvation survival kinetics
The kinetics of starvation survival of E. faecalis in water and nutrient-limited CDM are shown in Fig. 1. In water, both strains showed a dramatic drop in cell survival within the first 3 days of entering starvation, followed by a short period of adaptation and, thereafter, a relatively steady population cell density in very gradual decline. During prolonged starvation in water, both E. faecalis strains maintained a viable cell population of around 103 cfu/ml for more than 4 months, indicating the hardy starvation-survival capacity of this species.
Starvation in nutrient limited CDM revealed similar patterns of survival with a pronounced drop in cell numbers initially, followed by a prolonged period in which the cell density remained static. In phosphate-limited CDM, E. faecalis strain JH2-2 survived for >4 months (Fig. la), whereas E. faecalis strain OMGS 3264 showed a gradual decline in culturable cell numbers, eventually losing viability at 129 days (Fig. lb). In amino acid-limited CDM, E. faecalis strain JH2-2 lost viability at 35 days (Fig. la), whereas E. faecalis strain OMGS 3264 survived for >4 months (Fig. lb).
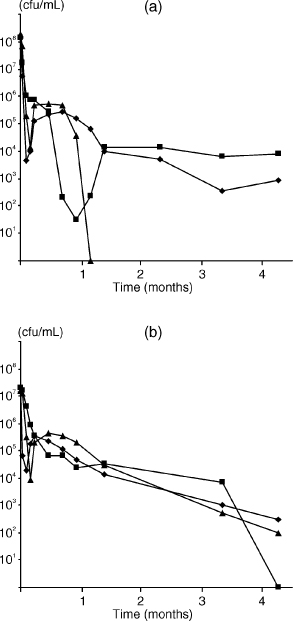
Fig. 1. Starvation-survival response of E. faecalis monitored for >4 months. Starvation survival kinetics in water (♦), phosphate-limited (■) and amino acid-limited (▲) CDM of E. faecalis (a) strain JH2-2 and (b) strain OMGS 3264.
Thus, both strains of E. faecalis survived in water for extended periods, but there was strain-specific variation for survival in nutrient-limited media.
Role of cell density and aerobic/anaerobic environment in starvation survival
The role of cell density in starvation survival is shown in Fig. 2. When cells were suspended in water at densities of 107 cfu/ml, E. faecalis strain JH2-2 lost viability after 3– 14 days. However, if the initial cell density was >108 cfu/ml at the onset of starvation, E. faecalis survived for prolonged periods (Fig. l and 2). Starvation cultures were mostly incubated at 37°C; however, some experiments were carried out at 25°C (data not shown), which resulted in slightly better survival than incubation at 37°C.
Because growth under anaerobic conditions may involve alternate metabolic pathways, we compared starvation survival of E. faecalis under anaerobic and aerobic conditions with a range of starting cell densities. Molecular analysis has shown differential expression of some genes for aerobic versus anaerobic growth in E. faecalis (47); however, we found no difference in the nature or duration of starvation survival (data not shown).
Growth of E. faecalis in serum and role of cell density
Initial growth experiments showed that there was no difference between 100% and 50% serum in supporting growth (data not shown). When E. faecalis was incubated in 50% PHS, cells were capable of growing and surviving for more than 4 months (Fig. 3). Interestingly, irrespective of the starting concentration (10–104 cells), E. faecalis grew to a density of around 106 cfu/ml within 2–4 days, with the population density remaining static thereafter for more than 4 months. These experiments were conducted with heat-inactivated serum. However, in some experiments cells were grown with untreated serum and there was no discernible difference in growth (data not shown).
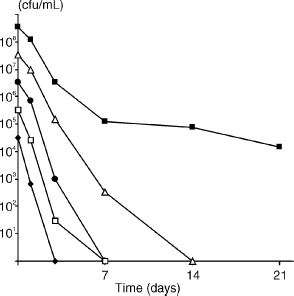
Fig. 2. Effect of cell density at the onset of starvation on survival of E. faecalis strain JH2-2, with aerobic incubation for 21 days. Starting cell densities were 3.3 × 108 (■), 3.3 × 107 (∆), 3.3 × 106 (●), 3.3 × 105 (□), 3.3 × 104 (♦) cfu/ml.
Fig. 3. Growth of E. faecalis strain JH2-2 in 50% serum for around 4 months. Starting cell densities were 5.1 × 104 (■), 5.0 × 103 (▲), 3.4 × 102 (△), 101 (⋄) cfu/ml.
A clear pattern of survival was observed for E. faecalis in minimal concentrations of serum, even when the initial cell density was low (Fig. 4). Regardless of the initial population numbers, both E. faecalis strains JH2-2 and OMGS 3264 showed similar growth characteristics in 1% and 5% serum (data not shown). These experiments demonstrated that as little 1% serum was sufficient for the survival and growth of low cell numbers of E. faecalis.
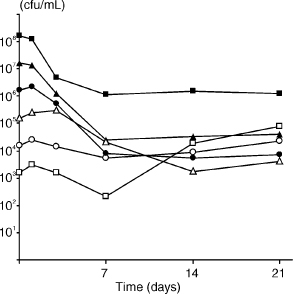
Fig. 4. Growth of E. faecalis strain JH2-2 in 1% serum for 21 days. Starting cell densities were 1.6 × 108 (■), 1.6 × 107 (µ), 1.6 × 106 (●;), 1.6 × 105 (△), 1.6 × 104 (▲), 1.6 × 103 (□) cfu/ml.
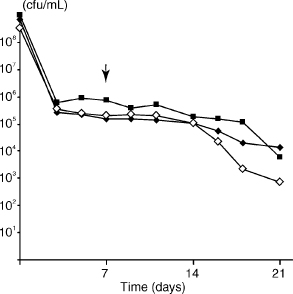
Fig. 5. Effect of inhibition of cell wall and DNA synthesis on starvation-state cells. Starvation of E. faecalis strain JH2-2 in water followed by addition of inhibitors on day 7 (↓): penicillin G (■), norfloxacin (■) and water controls (⋄). Cell densities at onset of starvation (day 0) were 1.0 × 109, 3.2 × 108, 6.9 × 108, respectively.
Metabolic activity of E. faecalis during starvation survival
When penicillin G was added to cells 7 days after onset of starvation, no reduction in viable cell numbers was observed for 11 days after inhibitor addition, compared with untreated cells. Fourteen days after inhibition of cell wall synthesis, there was a decline in viable cell numbers; the result of a representative experiment is shown in Fig. 5. On inhibition of DNA synthesis with norfloxacin, there was no change in cell viability during the first week; thereafter, there was a gradual decline in viable cell counts during the second week, compared to untreated starved cells (Fig. 5).
Revival of starved cells by serum
Addition of serum to starvation-state cells resulted in recovery and resumption of growth of E. faecalis (Fig. 6). Even when the number of starved cells was low (~10 cfu/ml), a serum fed recovery ensued, which was in contrast to controls where cells at densities below 104 cfu/ml lost viability after the addition of PBS.
Discussion
The species E. faecalis has adapted to a diverse range of nutritional environments—in its natural habitat in the human or animal gut it can benefit from a nutritionally rich milieu, yet as it leaves the digestive system it must endure periods of starvation if it is to survive and ultimately populate in a new host. This ability of E. faecalis to survive extended periods in nutritionally limited environments may be an important characteristic for the pathogenesis of E. faecalis in endodontic treatment failures. E. faecalis has frequently been recovered from failed cases where the root canal system had been obturated many years previously (45, 49), which would suggest an ability to endure a prolonged period of nutritional deprivation. As well as ensuring its survival for long periods, a starvation-induced survival state seems to confer some protection against a range of environmental stresses (13, 15, 16). Here, some features of starvation survival were characterized as well as growth of E. faecalis and recovery of starved cells in human serum. After the onset of nutrient limitation, the number of cultivable cells fell sharply in the first days. A small population survived, however, in a relatively static starvation state for many months. When starved cells encountered a nutritional upshift upon the addition of human serum, they were able to recover and to resume normal growth.
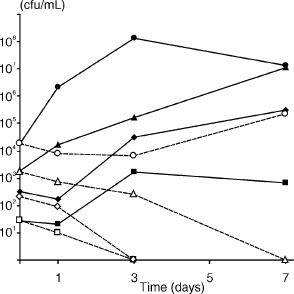
Fig. 6. Recovery of 7-day starvation-state cells of E. faecalis strain OMGS 3372 in 50% serum. After starvation for 7 days, serum (solid lines) or PBS (dashed lines) was added (shown as day 0) to starting cell densities of 1.8 × 104 (●), 1.8 × 103 (▲), 3.3 × 102 (♦), 2.7 × 101 (■) cfu/ml. In controls, PBS was added to equivalent starting cell densities (corresponding open symbols).
E. faecalis survived in nutrient-limited media for extended periods and endured starvation in water for more than 4 months. The number of cells at the start of starvation had a significant impact on starvation survival. When the cell density at the onset of starvation was <107 cfu/ml, loss of viability occurred after 3–14 days. However, when starvation commenced at a cell density >108 cfu/ml, E. faecalis survived for more than 4 months in water. These findings confirm other reports of starvation survival in water, where long-term survival of E. faecalis with a high cell density (>108 cfu/ml) at the onset of starvation (6, 20) has been observed, whereas starvation initiated with lower cell densities (~106 cfu/ml) resulted in a loss of viable cells in a much shorter period (31). A similar relationship between high initial cell density at the onset of nutrient limitation and starvation survival has also been reported for S. aureus (9, 51). The reason for the nexus between cell density and starvation survival may be found in studies that have investigated cell-to-cell communication and regulation of multi-cell activity.
Many genes are regulated by cell-density-induced signals (2, 10, 14, 46). Cell-to-cell communication allows a critical cell mass to be reached where the group can collectively achieve enhanced nutrition, or initiate alternative growth patterns when nutrition has been depleted (25, 27, 46). For example, in Bacillus subtilis, multiple signaling molecules are known to interactively sense and co-regulate cell-density, onset of starvation and sporulation (27). In this study, prolonged starvation survival of E. faecalis was dependent on a high cell density at the onset of starvation. Whilst cell-density signaling is implicated in the regulation of several virulence-related genes in E. faecalis (10, 11, 18, 41), it has yet to be determined whether signaling molecules are involved in the induction of a starvation-survival state.
Little is known about nutrients available in the apical part of a filled root canal. The nutrition required to sustain the microbial flora may come from fluid within dentinal tubules and/or fluid from the periapical tissues. As it is likely that the latter fluid is composed of a serum-like substance derived from surrounding tissue (28, 35), the growth and survival of E. faecalis was evaluated in human serum. In 50% serum, E. faecalis was able to survive for more than 4 months (Fig. 3). The observation that E. faecalis grew in serum is consistent with previous reports (1, 17, 28), but this is the first report of long-term survival of E. faecalis in serum. When E. faecalis was cultured in concentrations as low as 1% and 5% serum (Fig. 4), the growth values were comparable to those observed in higher serum concentrations. These experiments demonstrated that as little as 1% serum was sufficient for the growth and survival of very low numbers of E. faecalis, compared to complete nutrient deprivation in water where initial cell densities of >108 cells were necessary for survival.
The recovery of E. faecalis in serum (Fig. 6) may simply be associated with a nutritional upshift, but the involvement of growth-signaling molecules cannot be excluded. Some bacteria, such as Micrococcus luteus and Mycobacterium tuberculosis, enter a latent state under starvation or anoxic conditions (24, 52) and revival from this state is apparently induced by an extracellular resuscitation-promoting-factor, Rpf (3, 23, 36, 37). Starved cells of S. aureus are also revived by a factor in spent culture supernatant (7). It is unknown whether E. faecalis requires similar recovery signaling molecules, but a sequence search of the E. faecalis genome database shows no homologous sequence for the gene encoding the Rpf protein.
In the long-term growth experiment (Fig. 3), a population density of around 105 cfu/ml was reached within some days of culture in 50% serum and the population density remained static for more than 4 months, regardless of the initial cell concentration (10–104). Similarly, in experiments with as little as 1% serum, a population density of ~105 cfu/ml was reached within 7 days, irrespective of the starting cell concentration (Fig. 4). Although the mechanism for control of these events is unknown, it would not be unlikely if the management of such a well-regulated cell density were mediated by cell-cell communication.
There is general agreement from starvation-survival studies that bacteria in a nutrient-limited environment consist of three population groups—a decreasing number of starving (viable, but possibly injured) cells, non-culturable cells with intact cell membranes, and dead cells (including their constituents). It has been suggested that starvation under certain conditions produces a population of viable but not culturable (VBNC) cells that have the capacity for subsequent resuscitation, and some experimental data apparently support this view (42, 53). However, there is good evidence that cells which are seemingly VBNC are simply revived viable cells (4, 6).
A number of studies have purported to show a VBNC state in E. faecalis (29–32, 48). In some experiments (data not shown), we added serum to starvation cultures that had no apparent viable cells (106 cfu/ml-starting-density; starvation for 21 days). Surprisingly, an apparent ‘recovery’ of cells was observed; however, the effect was inconsistent and repeated attempts to reproduce resuscitation reliably were unproductive. There are two possible explanations for the ‘recovery’. One possibility is that some cells enter a VBNC state and can be revived by the addition of serum. A second, more likely explanation is that low numbers of injured cells survive starvation and are at the detection limit for viable plate counts, but the few remaining cells are enough for recovery to begin upon addition of serum. We intended to clarify whether or not E. faecalis enters a VBNC state, but it became evident from recent rigorous analysis that incontrovertible proof of the existence of a VBNC state is lacking (4, 6), and that authentication of a VBNC state cannot be achieved because current techniques are not necessarily reliable or accurate markers of viability (5).
Instead, we sought to clarify the condition of the starvation-state cells, which had been observed in static numbers for many months after the onset of nutrient limitation. The relatively constant density of starved cells could be due to a few active but ‘normal’ cells with a low rate of cell wall growth and division, or alternatively a small population of cells with minimal metabolic activity. If the static cell density were due to the former, then adding the cell-wall-synthesis inhibitor penicillin G, or the DNA synthesis inhibitor norfloxacin should hasten the death of the cells. When an inhibitory concentration of penicillin was added to starved cells there was minimal change in the rate of loss of viable cell numbers compared with untreated cells, whereas inhibition of DNA synthesis led to a slight fall in viable cell numbers (Fig. 5). Because the doubling time of growing E. faecalis cells is 65 min (6), the minor impact of these inhibitors on cell viability implies that starved cells were in a minimal metabolic state with limited cellular activity.
Taken together, these experiments show that E. faecalis is capable of enduring prolonged starvation and that starved cells can recover if serum is available—conditions that E. faecalis is likely to experience in many of its natural environments. If E. faecalis has an opportunity to access serum or serum-like fluid, even a small number of cells can gain the nutritional support required for survival. These characteristics are advantageous for E. faecalis to persist as a pathogen in the root canal—where a few cells may survive the antimicrobial measures during root canal treatment, be capable of multiplying in the presence of serum-derived nutrients, and therefore have the potential to maintain a periapical lesion.
Acknowledgements
We thank Mrs Chrissie Roth and Dr Priscilla Johanesen for technical assistance. This study was supported by grants from the Australian Society of Endodontology Inc, the Australian Society of Endodontology (Vic Branch) and the County Council of Västerbotten, Sweden.
References
1. Arduino RC, Murray BE, Rakita RM. Roles of antibodies and complement in phagocytic killing of enterococci. Infect Immun 1994: 62: 987–993.
2. Bassler BL. How bacteria talk to each other: regulation of gene expression by quorum sensing. Curr Opin Microbiol 1999: 2: 582–587.
3. Biketov S, Mukamolova GV, Potapov V, et al. Culturability of Mycobacterium tuberculosis cells isolated from murine macrophages: a bacterial growth factor promotes recovery. FEMS Immunol Med Microbiol 2000: 29: 233–240.
4. Bogosian G, Aardema ND, Bourneuf EV, Morris PJ, O’Neil JP. Recovery of hydrogen peroxide-sensitive culturable cells of Vibrio vulnificus gives the appearance of resuscitation from a viable but nonculturable state. J Bacteriol 2000: 182: 5070–5075.
5. Bogosian G, Bourneuf EV. A matter of bacterial life and death. EMBO Rep 2001: 2: 770–774.
6. Bogosian G, Morris PJ, O’Neil JP. A mixed culture recovery method indicates that enteric bacteria do not enter the viable but nonculturable state. Appl Environ Microbiol 1998: 64: 1736–1742.
7. Clements MO, Foster SJ. Starvation recovery of Staphylococcus aureus 8325-4. Microbiology 1998: 144: 1755–1763.
8. Dahlen G, Samuelsson W, Molander A, Reit C. Identification and antimicrobial susceptibility of enterococci isolated from the root canal. Oral Microbiol Immunol 2000: 15: 309–312.
9. Diaper JP, Edwards C. Survival of Staphylococcus aureus in lakewater monitored by flow cytometry. Microbiology 1994: 140: 35–42.
10. Dunny GM, Leonard BA, Hedberg PJ. Pheromone-inducible conjugation in Enterococcus faecalis: interbacterial and hostparasite chemical communication. J Bacteriol 1995: 177: 871–876.
11. Dunny GM, Leonard BA. Cell-cell communication in gram-positive bacteria. Annu Rev Microbiol 1997: 51: 527–564.
12. Fabricius L, Dahlén G, Holm SE, Möller AJR. Influence of combinations of oral bacteria on periapical tissues of monkeys. Scand J Dent Res 1982: 90: 200–206.
13. Flahaut S, Hartke A, Giard JC, Auffray Y. Alkaline stress response in Enterococcus faecalis: adaptation, cross-protection, and changes in protein synthesis. Appl Environ Microbiol 1997: 63: 812–814.
14. Fuqua C, Winans SC, Greenberg EP. Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum-sensing transcriptional regulators. Annu Rev Microbiol 1996: 50: 727–751.
15. Giard JC, Hartke A, Flahaut S, Benachour A, Boutibonnes P, Auffray Y. Starvation-induced multiresistance in Enterococcus faecalis JH2-2. Curr Microbiol 1996: 32: 264–271.
16. Giard JC, Hartke A, Flahaut S, Boutibonnes P, Auffray Y. Glucose starvation response in Enterococcus faecalis JH2-2: survival and protein analysis. Res Microbiol 1997: 148: 27–35.
17. Guzmàn CA, Pruzzo C, Platè M, Guardati MC, Calegari L. Serum dependent expression of Enterococcus faecalis adhesins involved in the colonization of heart cells. Microb Pathog 1991: 11: 399–409.
18. Haas W, Shepard BD, Gilmore MS. Two-component regulator of Enterococcus faecalis cytolysin responds to quorum-sensing autoinduction. Nature 2002: 415: 84–87.
19. Hancock HH, III, Sigurdsson A, Trope M, Moiseiwitsch J. Bacteria isolated after unsuccessful endodontic treatment in a North American population. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2001: 91: 579–586.
20. Hartke A, Giard JC, Laplace JM, Auffray Y. Survival of Enterococcus faecalis in an oligotrophy microcosm: changes in morphology, development of general stress resistance, and analysis of protein synthesis. Appl Environ Microbiol 1998: 64: 4238– 4245.
21. Hussain M, Hastings JG, White PJ. A chemically defined medium for slime production by coagulase-negative staphylococci. J Med Microbiol 1991: 34: 143–147.
22. Jacob AE, Hobbs SJ. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol 1974: 117: 360–372.
23. Kaprelyants AS, Kell DB. Do bacteria need to communicate with each other for growth? Trends Microbiol 1996: 4: 237–242.
24. Kell DB, Young M. Bacterial dormancy and culturability: the role of autocrine growth factors. Curr Opin Microbiol 2000: 3: 238–243.
25. de Kievit TR, Iglewski BH. Bacterial quorum sensing in pathogenic relationships. Infect Immun 2000: 68: 4839–4849.
26. Kjelleberg S, Albertson N, Flardh K, et al. How do non-differentiating bacteria adapt to starvation? Antonie Van Leeuwenhoek 1993: 63: 333–341.
27. Lazazzera BA. Quorum sensing and starvation: signals for entry into stationary phase. Curr Opin Microbiol 2000: 3: 177–182.
28. Love RM. Enterococcus faecalis – a mechanism for its role in endodontic failure. Int Endod J 2001: 34: 399–405.
29. del Mar Lleò M, Bonato B, Tafi MC, Signoretto C, Boaretti M, Canepari P. Resuscitation rate in different enterococcal species in the viable but non-culturable state. J Appl Microbiol 2001: 91: 1095–1102.
30. del Mar Lleò M, Pierobon S, Tafi MC, Signoretto C, Canepari P. mRNA detection by reverse transcription-PCR for monitoring viability over time in an Enterococcus faecalis viable but nonculturable population maintained in a laboratory microcosm. Appl Environ Microbiol 2000: 66: 4564–4567.
31. del Mar Lleò M, Tafi MC, Canepari P. Nonculturable Enterococcus faecalis cells are metabolically active and capable of resuming active growth. Syst Appl Microbiol 1998: 21: 333–339.
32. del Mar Lleò M, Tafi MC, Signoretto C, Dal Cero C, Canepari P. Competitive polymerase chain reaction for quantification of nonculturable Enterococcus faecalis cells in lake water. FEMS Microbiol Ecol 1999: 30: 345–353.
33. Molander A, Reit C, Dahlén G, Kvist T. Microbiological status of root-filled teeth with apical periodontitis. Int Endod J 1998: 31: 1–7.
34. Möller ÅJR. Microbiological examination of root canals and periapical tissues of human teeth. Methodological studies. Odontol Tidskr 1966: 74: 1–380.
35. Morse DR, Patnik JW, Schacterle GR. Electrophoretic differentiation of radicular cysts and granulomas. Oral Surg 1973: 35: 249–264.
36. Mukamolova GV, Kaprelyants AS, Young DI, Young M, Kell DB. A bacterial cytokine. Proc Natl Acad Sci USA 1998: 95: 8916–8921.
37. Mukamolova GV, Kormer SS, Kell DB, Kaprelyants AS. Stimulation of the multiplication of Micrococcus luteus by an autocrine growth factor. Arch Microbiol 1999: 172: 9–14.
38. Mukamolova GV, Yanopolskaya ND, Kell DB, Kaprelyants AS. On resuscitation from the dormant state of Micrococcus luteus. Antonie Van Leeuwenhoek 1998: 73: 237–243.
39. Nair PNR, Sjögren U, Figdor D, Sundqvist G. Persistent periapical radiolucencies of root-filled human teeth, failed endodontic treatments, and periapical scars. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999: 87: 617–627.
40. Nair PNR, Sjögren U, Krey G, Kahnberg KE, Sundqvist G. Intraradicular bacteria and fungi in root-filled, asymptomatic human teeth with therapy-resistant periapical lesions: a long-term light and electron microscopic follow-up study. J Endodon 1990: 16: 580–588.
41. Nakayama J, Cao Y, Horii T, Sakuda S, Akkermans AD, de Vos WM, Nagasawa H. Gelatinase biosynthesis-activating pheromone: a peptide lactone that mediates a quorum sensing in Enterococcus faecalis. Mol Microbiol 2001: 41: 145–154.
42. Oliver JD, Hite F, McDougald D, Andon NL, Simpson LM. Entry into, and resuscitation from, the viable but nonculturable state by Vibrio vulnificus in an estuarine environment. Appl Environ Microbiol 1995: 61: 2624–2630.
43. Ørstavik D, Haapasalo M. Disinfection by endodontic irrigants and dressings of experimentally infected dentinal tubules. Endod Dent Traumatol 1990: 6: 142–149.
44. Peciuliene V, Balciuniene I, Eriksen HM, Haapasalo M. Isolation of Enterococcus faecalis in previously root-filled canals in a Lithuanian population. J Endod 2000: 26: 593–595.
45. Peciuliene V, Reynaud AH, Balciuniene I, Haapasalo M. Isolation of yeasts and enteric bacteria in root-filled teeth with chronic apical periodontitis. Int Endod J 2001: 34: 429–434.
46. Schauder S, Bassler BL. The languages of bacteria. Genes Dev 2001: 15: 1468–1480.
47. Shepard BD, Gilmore MS. Identification of aerobically and anaerobically induced genes in Enterococcus faecalis by random arbitrarily primed PCR. Appl Environ Microbiol 1999: 65: 1470–1476.
48. Signoretto C, del Mar Lleò M, Tafi MC, Canepari P. Cell wall chemical composition of Enterococcus faecalis in the viable but nonculturable state. Appl Environ Microbiol 2000: 66: 1953–1959.
49. Sundqvist G, Figdor D, Persson S, Sjögren U. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1998: 85: 86–93.
50. Wai SN, Mizunoe Y, Yoshida S. How Vibrio cholerae survive during starvation. FEMS Microbiol Lett 1999: 180: 123–131.
51. Watson SP, Clements MO, Foster SJ. Characterization of the starvation-survival response of Staphylococcus aureus. J Bacteriol 1998: 180: 1750–1758.
52. Wayne LG. Dormancy of Mycobacterium tuberculosis and latency of disease. Eur J Clin Microbiol Infect Dis 1994: 13: 908– 914.
53. Whitesides M, Oliver J. Resuscitation of Vibrio vulnificus from the viable but nonculturable state. Appl Environ Microbiol 1997: 63: 1002–1005.