Cell surface structures of Actinomyces israelii
David Figdor, MDSc, LDS, FRACDS, Dip Endo*
John Davies, BSc, PhD†
Abstract
Actinomyces israelii is the most common cause of human actinomycosis, a chronic granulomatous infection. Periapical actinomycosis involving A. israelii has been identified as an important cause of failure of conventional endodontic treatment. Structures on the bacterial cell surface have been implicated in the pathogenicity of Actinomyces. In this study the ultrastructure of A. israelii was investigated by electron microscopy. Negatively stained preparations revealed the presence of hairlike fimbriae protruding through a thick surface coat on some species, whilst thin sectioning disclosed a Gram-positive cell wall surrounded by a fuzzy outer coat. These structures may be important for the pathogenicity of A. israelii.
Key words: Actinomyces israelii, fimbriae, pathogenicity, ultrastructure.
(Received for publication June 1996. Revised August 1996. Accepted September 1996.)
Introduction
Microorganisms play an essential role in the aetiology and development of inflammatory processes in the periapical tissues resulting in apical periodontitis.1,2 Although endodontic treatment is successful in the majority of cases, about 10 per cent of well-treated cases end in failure.3,4 Recent investigations, which have combined advanced microbiological techniques with sophisticated light and electron microscopic analysis, have shown that there are four factors which may individually or collectively contribute to endodontic failure. The most important and commonly occurring factor is a persisting infection within the root canal system.5 Other causes of failure involve foreign body reactions which impair healing6 and true cysts containing cholesterol crystals.7 The fourth factor, revealed in well documented cases, is a persisting extraradicular infection of the periapical tissues that results in failure of endodontic therapy.8-10
Bacteria of the species Actinomyces israelii have been repeatedly identified in cases of failed endodontic therapy causing a persistent extraradicular infection in the tissues, or periapical actinomycosis.8-11 Actinomyces israelii is the most commonly isolated species in actinomycosis, causing a chronic infection which is often resistant to clinical treatment. In cases of periapical actinomycosis the preferred treatment method is surgical curettage, since clinical reports12,13 and laboratory studies14 show that antibiotics are only likely to be effective when administered for prolonged periods.
A characteristic feature of infection by A. israelii is the ability of the bacteria to establish in tissue and survive the host defence, yet little is known about the pathogenic mechanisms by which A. israelii is able to evade the host defence. In a previous study15 the pathogenicity of A. israelii was examined using a strain which had been isolated from a case of failed endodontic therapy.10 This strain was able to establish itself in an experimental infection model in guinea pigs in spite of the host defence. This finding implied an impaired function of the host phagocytes, yet in vitro experiments showed that this strain was easily phagocytosed and killed by human polymorphonuclear leucocytes (PMNs). It seems that the ability of A. israelii to survive in the face of an effective host defence is associated with the ability of these bacteria to build cohesive colonies of branching filaments in vivo which enable them to collectively escape destruction by host defence systems. That the cells of A. israelii aggregate so cohesively, suggests the involvement of structures on the cell surface of these bacteria. The aim of this study, therefore, was to examine the ultrastructural features of A. israelii so as to identify structural elements which might contribute to the pathogenicity of A. israelii.
Materials and methods
Bacteria and growth conditions
Two strains of A. israelii were stored at -70°C in 20 per cent skim milk until required for use. Both were originally from human clinical material and designated AH and L110B. The AH strain‡ was isolated from the periapical tissue of an endodontic therapy-resistant case10 and strain L110B§ was isolated from a deep carious lesion.16 The strains were grown on 1 per cent brain heart infusion agar,’ placed in anaerobic jars with a palladium catalyst in a gas mixture of 10 per cent CO2 and 10 per cent H2 in nitrogen and incubated at 37°C. The strains were grown for at least 7 days by which time the size of both AH and L110B colonies varied between 1-1.5 mm in diameter. AH colonies were pale yellow with a rough surface, and corroded the agar. L110B colonies were cream coloured with a fluffy rough surface, and showed little tendency to pit the agar surface. Both strains were confirmed as A. israelii using a biochemical test kit¶ and classified according to the VPI Anaerobe Laboratory Manual.17
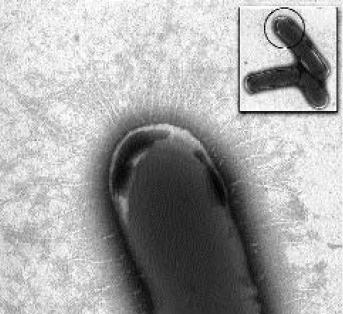
Fig. 1. – An electron microscope view of a whole cell preparation of Actinomyces israelii, strain L110B. Main picture shows a highly magnified view of the circular demarcated area of one of the three bacteria in the rectangular inset. The hairlike fimbriae can be seen protruding through a fuzzy coat which covers the entire surface of the cell. × 40 000; inset × 6760.
Electron microscopy
Bacteria were processed and examined as whole cells after negative-staining and as thin sectioned material after plastic-embedding. For negative stained preparations, cells were harvested, suspended in sterile phosphate buffered saline (PBS), washed twice with gentle manual agitation and then resuspended in 100µL PBS. Bacterial cell suspensions were placed on copper grids and the cells stained with 0.5 per cent phosphotungstic acid, pH 7, for 10 s. These negatively stained whole cell preparations were examined and micrographed in a transmission electron microscope (TEM).**
The thin sectioned bacterial cells were prepared using a method similar to that described previously.18 Briefly, colony-containing BHI agar plates we re overlaid with BHI broth supplemented with 2.5 per cent sodium chloride and 0.6 per cent agar. When set, agar embedded colonies were dissected from the plates and stored overnight at 4°C in fixative (2.5 per cent glutaraldehyde in 0.1 mol/L cacodylate buffer containing 0.1 mol/L sucrose). Some agarembedded colonies were additionally treated by adding 0.1 per cent ruthenium red, which enhances the staining of the outer coat, to the fixative during storage overnight at 4°C. Thereafter, the specimens were washed, osmicated, dehydrated through a graded series of ethanol, embedded in resin,†† thin sectioned, contrasted in uranium and lead salts and examined in a TEM.**
Results
Electron microscopy of negatively stained whole-cell preparations showed that the body of the bacteria had a mottled appearance and the electron lucent inner aspect of the cell wall revealed irregular patches of electron-dense material. The cell surface was covered with a thick, fuzzy coat (Fig. 1). On some cells of strain L110B, hairlike structures could be seen protruding through the fuzzy outer coat. These hairy structures were evenly distributed over the entire surface of the bacterial cell wall (Fig. 1).
Electron microscopy of thin sectioned colonies revealed masses of tightly packed bacteria. Most cells revealed an amorphous but granular cytoplasm (Fig. 2). In some sections, septation was evident between cells indicating bacterial growth by cell division. Individual cells disclosed wall characteristics of Gram-positive bacteria. Wrapped around the Gram-positive walls of individual bacteria was an electrondense matrix which conformed with the fuzzy outer coat described for whole-cell preparations. The fuzzy coat was thinner on strain L110B and thicker on strain AH, and on the latter often resembled sheet-like material folded around and packed between the cells, so that the matrix appeared to bind the cells together (Fig. 2, inset).
Discussion
Many determinants of pathogenicity are located around the bacterial cell surface, so that identification of physical structures by electron microscopy can provide useful information about the mechanisms of pathogenicity. A significant finding in this study was the presence of hairy fimbriae-like structures in a fuzzy outer coat which indicates that at least some strains of A. israelii have the capacity to produce fimbriae.
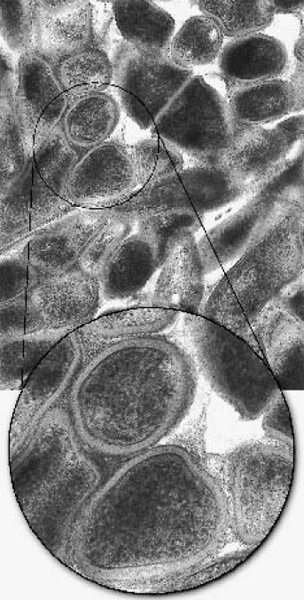
Fig. 2. – Fine structure of thin sectioned colony of Actinomyces israelii, strain AH, without ruthenium red staining. Note the tightly packed cells revealing a Gram-positive wall structure (inset) and the fuzzy outer coat wrapped around and between individual cells. × 22352; inset × 50 400.
Adhesion of bacteria to host tissues is a vital step preceding the establishment of infection by pathogenic bacteria.19 Fimbriae play an important role in many infections by mediating adhesion to various host cells and surfaces. Most descriptions of the presence and role of fimbriae have focused on Gram-negative bacteria in which the molecular biology of production, assembly and expression as well as the character and function of their adhesins in colonization has been reasonably well characterized.20 There has been relatively little investigation of fimbriae on Gram-positive bacteria. However, two other oral Actinomyces species, A. viscosus and A. naeslundii, have been shown to possess fimbriae on their cell surface.21,22 The fimbriae on A. viscosus and A. naeslundii have been differentiated into two types on the basis of their adhesive properties and molecular structure. The type 1 fimbriae on A. viscosus mediate bacterial attachment to the tooth surface and salivacoated hydroxyapatite and the type 2 fimbriae are associated with a lectin activity that mediates adhesion to epithelial cells and PMNs,23-25 as well as coaggregation with other bacteria.26
The hairlike structures reported in this study strongly resemble structures identified as fimbriae on various bacteria20 and on other Actinomyces species.22 After identifying fimbriae on strain L110B by electron microscopy, an attempt was made to remove and harvest fimbriae from this strain by a variety of physical and chemical techniques, however these methods were unsuccessful. The authors therefore sought to identify the fimbriae on A. israelii using a genetic approach. A DNA probe was constructed, based on a conserved region of the genes encoding type 1 and 2 fimbriae in A. viscosus and A. naeslundii.27 The fimbrial gene in A. israelii could not be identified using this approach, a finding which corroborates recent similar work28 and suggests that the fimbriae on the surface of A. israelii represent a different type from that found on the comparatively well characterized A. viscosus and A. naeslundii.
These results show that fimbriae-like structures are present on some strains of A. israelii but they appear to differ from the type found on related Actinomyces species.21,22 Both the fimbriae and the matrix of the outer coat surrounding the bacteria may help the cells to aggregate into cohesive colonies of tangled filaments, an ability which has been suggested15 to be vital for the survival of A. israelii in host tissues.
Acknowledgements
The authors thank Mrs Khim Hoe for assistance with the electron microscopy and Dr Ramachandran Nair, Switzerland for valuable criticism of the manuscript. This work was supported by grants from the Australian Dental Research Fund Inc. (now Foundation), the Australian Society of Endodontology (Victorian Branch) and the Australian Society of Endodontology Inc.
References
1. Kakehashi S, Stanley HR, Fitzgerald RJ. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol 1965;20:340-9.
2. Sundqvist G. Bacteriological studies of necrotic dental pulps. Umeå, Sweden: University of Umeå, 1976. Odontological Dissertation No. 7.
3. Sjögren U, Hägglund B, Sundqvist G, Wing K. Factors affecting the long-term results of endodontic treatment. J Endod 1990;16:498-504.
4. Sjögren U. Success and failure in endodontics. Umeå, Sweden: University of Umeå, 1996. Odontological Dissertation No. 60.
5. Nair PNR, Sjögren U, Krey G, Kahnberg K-E, Sundqvist G. Intraradicular bacteria and fungi in root-filled asymptomatic human teeth with therapy-resistant periapical lesions: a long-term light and electron microscopic follow-up study. J Endod 1990;16:580-8.
6. Nair PNR, Sjögren U, Krey G, Sundqvist G. Therapy-resistant foreign body giant cell granuloma at the periapex of a root-filled human tooth. J Endod 1990;16:589-95.
7. Nair PNR, Sjögren U, Schumacher E, Sundqvist G. Radicular cyst affecting a root-filled human tooth: a long-term post-treatment follow-up. Int Endod J 1993;26:225-33.
8. Byström A, Happonen R-P, Sjögren U, Sundqvist G. Healing of periapical lesions of pulpless teeth after endodontic treatment with controlled asepsis. Endod Dent Traumatol 1987;3:58-63.
9. Happonen R-P. Periapical actinomycosis: a follow-up study of 16 surgically treated cases. Endod Dent Traumatol 1986;2:205-9.
10. Sundqvist G, Reuterving C-O. Isolation of Actinomyces israelii from periapical lesion. J Endod 1980;6:602-6.
11. O’Grady JF, Reade PC. Periapical actinomycosis involving Actinomyces israelii. J Endod 1988;14:147-9.
12. Rippon JW. Medical Mycology. 3rd edn. 2. Actinomycosis. Philadelphia: Saunders, 1988:30-53.
13. Peabody JW Jr, Seabury JH. Actinomycosis and nocardiosis. A review of basic differences in therapy. Am J Med 1960;28:99-115.
14. Barnard D, Davies J, Figdor D. Susceptibility of Actinomyces israelii to antibiotics, sodium hypochlorite and calcium hydroxide. Int Endod J 1996;29:320-6.
15. Figdor D, Sjögren U, Sorlin S, Sundqvist G, Nair PNR. Pathogenicity of Actinomyces israelii and Arachnia propionica: experimental infection in guinea pigs and phagocytosis and intra-cellular killing by human polymorphonuclear leukocytes in vitro. Oral Microbiol Immunol 1992;7:129-36.
16. Edwardsson S. Bacteriological studies on deep areas of carious dentine. Odont Revy 1974;25:Suppl 32.
17. Holdeman LV, Cato EP, Moore WEC. Anaerobe laboratory manual. 4th edn. Blacksburg, Virginia, USA: VPI Anaerobe Laboratory, Virginia Polytechnic Institute and State University, 1977.
18. Sundqvist G, Figdor D, Hänström L, Sörlin S, Sandström G. Phagocytosis and virulence of different strains of Porphyromonas gingivalis. Scand J Dent Res 1991;99:117-29.
19. Beachey EH. Bacterial adherence: Adhesin-receptor interactions mediating the attachment of bacteria to mucosal surfaces. J Infect Dis 1981;143:325-45.
20. Klemm P. Fimbriae. Adhesion, genetics, biogenesis and vaccines. Boca Raton: CRC Press, 1994.
21. Girard AE, Jacius BH. Ultrastructure of Actinomyces viscosus and Actinomyces naeslundii. Arch Oral Biol 1974;19:71-9.
22. Lai C-H, Listgarten MA. Immune labeling of certain strains of Actinomyces viscosus and Actinomyces naeslundii by fluorescence and electron microscopy. Infect Immun 1979;25:1016-28.
23. Cisar JO. Fimbrial lectins of the oral Actinomyces. In Mirelman D, ed. Microbial lectins and agglutinins. New York: Wiley, 1986:183-96.
24. Cisar JO, Sandberg AL, Clark WB. Molecular aspects of adherence of Actinomyces viscosus and Actinomyces naeslundii to oral surfaces. J Dent Res 1989;68:1558-9.
25. Strömberg N, Borén T. Actinomyces tissue specificity may depend on differences in receptor specificity for GalNAcβ-containing glycoconjugates. Infect Immun 1992;60:3268-77.
26. Kolenbrander PE, London J. Minireview. Adhere today, here tomorrow: Oral bacterial adherence. J Bacteriol 1993;175:3247-52.
27. Yeung MK, Cisar JO. Sequence homology between the subunits of two immunologically and functionally distinct types of fimbriae of Actinomyces spp. J Bacteriol 1990;172:2462-8.
28. Yeung MK. Conservation of an Actinomyces viscosus T14V type 1 fimbrial subunit homolog among divergent groups of Actinomyces spp. Infect Immun 1992;60:1047-54.
Address for correspondence and reprints:
Dr D. Figdor,
Medical Centre,
517 St Kilda Road,
Melbourne, Victoria 3004,
Australia.
*Visiting Scientist, Department of Microbiology, Faculty of Medicine, Monash University.
†Associate Professor, Department of Microbiology, Faculty of Medicine, Monash University
‡Kindly provided by Professor G. Sundqvist, University of Umeå, Sweden.
§Kindly provided by Professor S. Edwardsson, Lund University, Sweden.
,BHI Broth and No. 1 Agar. Oxoid, Basingstoke, England.
¶Microbact System 24AN. Pacific Diagnostics, Adelaide, South Australia.
**JEM-100S. JEOL, Tokyo, Japan.
††Spurr resin. Agar Aids, Essex, UK.