Ann R Aust Coll Dent Surg 1994;12:131-42.
SEMINAR
ASPECTS OF DENTINAL AND PULPAL PAIN
PAIN OF DENTINAL AND PULPAL ORIGIN —
A REVIEW FOR THE CLINICIAN*
David Figdor, MDSc, LDS, FRACDS, Dip Endo†
ABSTRACT
Recent advances in understanding the mechanisms of pain arising from the dental pulp serve to benefit patients by improving the clinician’s ability to diagnose and treat pain. There are two types of pain arising from the pulp which are mediated by entirely different nerve fibres, each with their own individual characteristics. One is a short, sharp fast pain which is induced by stimuli which cause a rapid fluid flow within the dentinal tubules. Such stimuli include cold, heat, air, drilling, and osmotic stimuli. Once the affected teeth are identified, they can often be treated by sealing the open, exposed dentine. The second type of pain is experienced as a slow, dull, aching, poorly localized pain which is mediated by pain fibres activated by stimuli which are noxious to the pulp, such as prolonged damaging heat and inflammatory mediators. Pain of this character can be difficult to diagnose and often indicates serious pulp damage necessitating removal of the offending pulp by endodontic therapy.
INTRODUCTION
Since dentistry was first practised, the prime reason for those seeking some form of dental treatment has been for the relief of pain. For patients, the elimination of pain, understandably, takes precedence over all other reasons for presentation to the dentist and as a profession, the relief of oral pain is of the highest priority. And yet, despite this, the elucidation of the mechanisms of oral pain has remained largely obscure until the last three decades when advances in neurophysiology have made oral pain easier to understand and treat.
Pain is no longer conceptualized in the limited sense of a sensory response to a noxious stimulus, but is now regarded by researchers as a multifactorial experience which may be modified by a variety of cognitive, emotional and motivational influences.1 Experienced clinicians will recognize this statement as an intuitive truth, for it means that a patient who presents with pain will convey not only the sensory event, but the sum of the sensation and its modification by their own of culture, memory, anxiety, emotion, motivation and personality.1
The dental pulp has for a long time been thought of as a richly innervated tissue which, when stimulated, has a single sensory response — pain. Recent evidence suggests that the sensory response can be divided into three parts according to the type and intensity of the stimulus. When an electric stimulus of low frequency and current is experimentally applied to a human tooth a sensation of pre-pain is perceived.2,3 An increase in the electrical current or frequency, or the application of intense cold or heat to an intact tooth can induce an initial rapid, sharp pain.2,3 The clinician will recognize this as analogous to the presentation of a patient with exposed dentine who describes a short, sharp pain brought on by cold air, drinking hot or cold beverages or sweet foods. A second, delayed dull pain can be experimentally induced by strong electrical stimulation or intense heating or cooling4 which reaches the pulp proper.2,3 This is equivalent to the familiar clinical presentation of a painful pulpitis with a dull, aching, poorly localized pain, often aggravated by hot drinks and which may have a duration of some hours. The recognition of the existence of these three components of dental pain and the nature of the nerve fibres which carry them is an important milestone for the researcher and clinician.
It is only through an improved understanding of the mechanisms of such pain that the conscientious clinician can provide a high level of clinicial diagnosis and appropriate clinical treatment. The purpose of this paper is to provide a review of neurophysiological advances of dental pain which are of relevance to clinical dentistry.
MECHANISMS OF PAIN ACTIVATION AND TRANSMISSION
Pain theories — How are pain fibres activated in the dental pulp?
For the conscious perception of pain, sensory nerve fibres within the pulp must be activated which then transmit impulses centrally to the brain. The mechanism of activation of pain within the pulp has been the subject of some debate over many years and during this time three theories of pain activation have been proposed.
The Conduction theory5 is based on the notion that exposed dentine is sensitive to stimuli due to direct activation of nerve fibres within the dentinal tubules. There is very little support for this concept since numerous light and electron microscopic investigations have failed to convincingly show nerve fibres extending more than a short distance into peripheral dentine.6 Furthermore, application of local anaesthetic solution, or pain inducing chemicals, to cut dentine does not block nerve activity or cause pain,7,8 respectively, which would be expected if nerve fibres were indeed present in the exposed dentine.
The Transduction theory9 proposes that the odontoblast process itself acts as a receptor and transducer and that pain impulses are then passed to nerve fibres located around the odontoblast cell body. Once again, there is little to substantiate this concept since the odontoblast process does not extend more than about one-third of the way to the dentino-enamel junction.10,11 There is virtually no ultrastructural evidence of specialized tight junctions between the nerve fibres and the odontoblast cell body12 which would be necessary if pain impulses were to be transmitted from the odontoblast to the nerve itself. Further convincing evidence against this theory is that in those cases where the odontoblasts are destroyed or missing the dentine has been shown to remain sensitive.13,14
Undoubtedly, the theory which has the most credence is the Hydrodynamic theory originally proposed by Gysi at the turn of the century and revived and experimentally substantiated by Brännström in a series of remarkable experiments which began more than 30 years ago and which have been the subject of excellent reviews.15,16 The Hydrodynamic theory is based on the relatively simple concept that rapid movement of fluid within the dentinal tubules induces pain. The dentinal tubules contain a fluid, an ultrafiltrate of blood from capillaries within the pulp, which under normal conditions moves outward at a very slow rate.17,18 If this fluid is made to move rapidly through the tubule, pain fibres located around the odontoblast process and at the pulp-dentine border are activated.19 Whilst there is overwhelming evidence in support of the hydrodynamic theory, it is now understood that this accounts for only one of the two types of painful sensations arising within the pulp. Nevertheless, the confirmation of this theory has been a significant milestone in the understanding and treatment of dentinal pain.
Nerve fibres within the dental pulp
With the advent of research utilizing sophisticated single nerve fibre recording methods along with good ultrastructural investigations, great strides have been made which provide a more complete picture of the origin of pain impulses within the pulp. In the sixties, considerable research effort was expended trying to correlate the clinical features of a painful pulp and the histological picture associated with such pulps. These efforts were unsuccessful since there was little correlation between the histology and the clinical symptoms.20 It is now clear that in part, this is due to the fact that there are two distinctly different types of nerve fibres within the pulp which are activated in separate ways; one of which bears a high correlation with the condition of the dentine surface21 rather than the condition of the underlying pulp itself.22
The dental pulp is supplied with both myelinated and non-myelinated nerve fibres.23-25 The classical description of non-motor nerve fibres has been related to their functions: sensory and autonomic — sympathetic (which is known to operate within the dental pulp26) and parasympathetic. This was predicated on the assumption that these nervous pathways served essentially separate functions; however, recent evidence suggests that their ‘separate’ roles are far less distinct than were previously thought.27 It now appears that afferent fibres which would previously have been deemed to be purely sensory are now thought to play a supplementary role such as a vasomotor function to regulate blood flow within the pulp.27 However, the function of these nerves is still considered to be predominantly sensory.
A clinically useful way of dealing with pain arising from the pulp is according to the types of sensory nerve fibres present within the pulp. Nerve fibres are classified according to their diameter and conduction velocity.24,25,28 Within the dental pulp, three types of pain fibres have been identified — two have the protective myelin sheath and one is unmyelinated.23-25 The myelinated A-beta fibres have a larger axon diameter (>5 μm)25 and faster conduction velocity of ≥30 m/s,2 compared with A-delta fibres with an average 3.5 μm diameter25 and <30 m/s conduction velocity.2 The unmyelinated C-fibres have an axon diameter of ≤0.5 μm23 and conduction velocity of ≤2 m/s.2,28 Each of these fibres have an important role in the experience and perception of pain. The characteristics of the A- and C-fibre nerves are fundamentally different and are described in detail below.
A-fibres
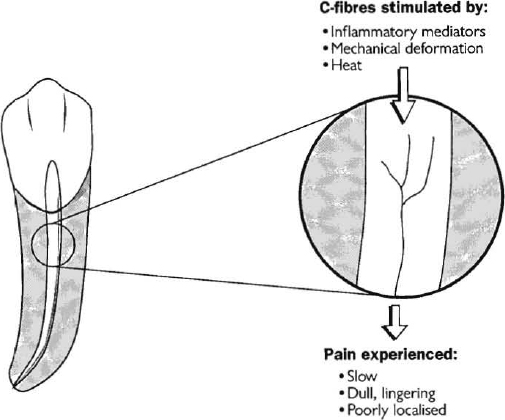
About 93 per cent of the myelinated fibres entering the human premolar pulp are in the A-delta range while the remaining 7 per cent are of A-beta variety.25 The A-beta fibres are thought to be functionally similar to the A-delta fibres3 (and will therefore be considered as one group in this paper), although A-beta fibres have been reported to have a slightly different character in that they can respond to vibration29 and are stimulated at a lower electrical threshold.2 The site which responds to stimulation, or receptive field, of these fibres is located at the pulp-dentine border or in the dentine.30,31 These fibres have mechanically sensitive receptor endings located in close proximity to the odontoblast cell body.12,18 The A-delta fibres are activated by a hydrodynamic mechanism in which there is a sudden movement of fluid within the tubules15 which stimulates the mechanosensitive nerve ending resulting in a short, sharp initial pain.32-35 The principal clinical features of the A-delta fibres are that they are activated by hydrodynamic stimuli, such as drilling, sweet foods, air and cold, which leads to rapid fluid movement within the tubules. A summary of the principal features of these fibres is found in Fig. 1.
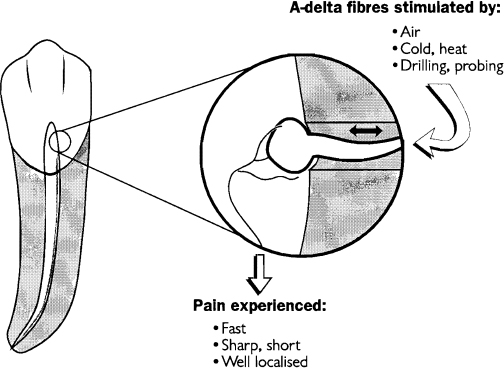
Fig. 1.—Diagram illustrating the characteristics of A-delta nerve fibres within the dental pulp. Magnified view shows the odontoblast lying within the dentinal tubule and the mechanically sensitive A-delta nerve ending which surrounds the odontoblast cell body.
A logical consequence of their method of activation is that any factors which might lead to a potential increase in fluid movement, by opening dentinal tubules, would lead to an increase in dentine sensitivity.36 Clinical examples of this are cutting a cavity and exposure of open root dentine by scaling or toothbrush abrasion.
C-fibres
About 70-80 per cent of nerve axons entering the pulp are unmyelinated.3 It is unknown what proportion of these are sensory C-fibres. These fibres are functionally different from A-fibres; they are slow conducting, produce a dull, poorly localized sensation and are activated by inflammation, heat and mechanical deformation.3,30,37,38 The receptive field of these fibres is located deep in the pulpal tissue.30,31 A summary of their features can be found in Fig. 2. The typical clinical description of this type of pain is that it is a dull, vaguely located, aching pain which increases several seconds after a hot drink.
Fig. 2.—Diagram illustrating the characteristics of C-fibres within the dental pulp. Magnified view shows the nerve fibre within the pulp.
The C-fibres are usually activated by stimuli which cause actual damage to pulp tissue; thus these nerves respond to inflammatory mediators released during the process of pulp inflammation and to prolonged hot stimuli.3,39 These fibres are also resistant to tissue anoxia, so that under conditions in which the A-delta fibres have ceased to respond due to decreased pulpal blood flow and poor oxygenation, the C-fibres retain their capacity to be activated.3,28
The ability of the C-fibres to survive under adverse conditions where much of the normal pulp tissue has been destroyed is more than likely the reason for a familiar clinical occurrence in which a tooth, which responds negatively to testing with a cold CO2 stick (since the A-delta fibres have degenerated), is undeniably painful to mechanical instrumentation at the commencement of endodontic therapy. Therefore, the cautious practitioner who seeks to avoid pain during root canal therapy would be wise to administer a local anaesthetic in those cases where a patient reports pain to hot drinks (indicating viable C-fibres) and the tooth responds negatively to cold or electric sensitivity tests.
APPLICATION TO CLINICAL DIAGNOSIS AND TREATMENT
Stimuli and the healthy pulp
Under normal conditions, a tooth with a healthy functioning pulp is sensitive to intense hot and cold stimuli. Such stimuli cause a rapid movement of fluid within the dentinal tubules16,40 which activates the mechanically sensitive receptors of the A-delta fibres resulting in the experience of an initial, sharp pain of a short duration of one to several seconds. Equally, other stimuli such as air blasts, drilling and osmotic stimuli (such as sweet foods) will induce a similar response.3
When a ‘vitality’ test is performed, usually with the application of a cold or electric stimulus, only the A-delta fibres are normally activated. It should be understood that such a test is actually a sensitivity test in which a positive response provides information solely related to the activation and competent functioning of A-delta fibres. It conveys no information regarding C-fibres or about the blood supply and vitality of the pulp tissue itself.
Under ordinary conditions, C-fibres are not activated in the healthy pulp, although under certain experimental conditions a prolonged hot38 or cold31,41 stimulus, or high intensity electrical stimulation28 can produce a dull pain through the recruitment of some C-fibres deep within the pulp.
Why is the tooth sensitive following restoration?
For a long time it was though that pain, which occurred following the placement of an amalgam restoration, was due to the transfer of heat to the pulp by thermal conductivity through the metallic restoration.42 This concept was erroneous, since we now know that the mechanism of activation of such pain is by the hydrodynamic movement of fluid within the dentinal tubules. There is an increased capacity for fluid to move through open dentinal tubules after the placement of an amalgam or composite restoration since neither restoration is capable of producing a perfect fluid-tight seal of the cavity.43-45 In fact, what occurs in most cases is the formation of a microscopic gap between the filling and the cavity wall which permits the ingress of fluid (and bacteria) into the space. This phenomenon of fluid moving into and around the gap is termed microleakage and it has important implications for pulpal health43,46-48 and postoperative sensitivity.49 If the gap is open, then a continuous fluid passage from the pulp through to the oral cavity may exist. Under these conditions application of stimuli, which have the potential to cause a rapid fluid flow through dentinal tubules, will result in a sensitive tooth.49 The modern management of cavities prepared in dentine (without pulp exposure) involves not thick bases for ‘thermal insultation’, but methods to seal the open dentinal tubules through the use of oxalates,45,50,51 double liners (Tubulitec)52 or resins,53,54
Permeable dentine and the smear layer
The smear layer is another factor of great relevance for dentinal sensitivity. The smear layer is a mineralized dentine matrix which adheres tenaciously to the dentine surface and is produced whenever dentine is cut.55 It is particularly important in this context because of its effect on dentine permeability.56 Permeability of dentine is defined as the ability of fluids and substances to move through dentine. There is good evidence to show that the presence of a smear layer on cut dentine can reduce dentine permeability by approximately 5- to 10-fold.56-58 In some respects, this would seem to be advantageous since a reduction in dentine permeability leads to the potential for decreased fluid flow and thus less sensitivity to hydrodynamic stimuli. However, we know that the smear layer can be very quickly degraded by acids:56 those used during restorative procedures (such as acid etching); food acids; or organic acids produced by micro-organisms that have invaded (or been left in) the gap between the restoration and the tooth. When these acids break down the smear layer, there can be an enormous increase in dentine permeability and a consequential enhancement of sensitivity.33
It is no wonder then that the placement of an acid etched composite can lead to a very sensitive tooth. In these circumstances, there is the combined problem of a fluid-filled contraction gap between the composite material and the cavity wall,59,60 as well as the likelihood of the smear layer being removed by an acid etchant in the restorative procedure,56 and deformation of the composite resin under load.61 The result is permeable, open dentinal tubules which have a continuous fluid connection from the pulp through to the oral environment and thus the potential for rapid fluid flow and significant postoperative sensitivity.44
The realization that dentine permeability is so significant to pulpal health and dentinal pain is a good example of a circumstance where basic in vitro and in vivo research has led to substantial improvements in the method for treatment of real clinical problems. For the reliable prevention of post-restorative sensitivity, and treatment of dentinal sensitivity generally, every attempt should be made to occlude exposed or open dentinal tubules.49,62
What happens in the inflamed pulp?
There is a considerable variation in the presentation of patients with a painful pulpitis which often makes diagnosis difficult for the clinician. In the inflamed pulp, an intricate set of interactions contribute to make the pain vary from something mild to occasionally a debilitating and severe pain. Whilst, progress is being made to understand the processes involved, these factors can be summarized as events which may occur between the sensory nerves and one or several of:
1. Blood flow and pulpal tissue pressure. The sensory nerves are capable of altering pulpal blood flow;27,63-65 conversely, alterations in blood flow (which affect oxygen tension and local tissue pressure66) can alter nerve excitability.67-70
2. Bacteria and their metabolites. Bacteria can physically block dentinal tubules,71 alternatively, they can expose and open tubules through the production of acids.72,73 Some bacterial metabolites have direct effects on nerves.72,73
3. Inflammation and inflammatory mediators. A whole host of inflammatory mediators have been identified and many of these produce pain by directly activating (and possibly altering the morphology of74) the C-fibres.28,68 However, at the present time only one mediator (serotonin35,37,76) is known to have the capacity to induce pain in A-delta fibres. Indirect effects of inflammatory mediators on pain may also occur through the ability of some mediators to alter pulpal blood flow and to potentiate other substances which affect the sensitivity of pulp nerves.
4. Permeability of the dentine. As discussed previously the tubules may be open, which can induce sensitivity77,78 by directly affecting the A-delta fibres,79,80 and may affect the health of the pulp by providing an open pathway for microbial invasion. The tubules can also be occluded anywhere along their length from the pulp side, intratubularly or on the oral cavity side.
It is useful to make some distinctions between patients who present with pain, on the basis of the preceding information about sensory nerve fibres. A variety of terms have previously been used to describe the state of the pulp for the purposes of clinical diagnosis, most of which have had limited functional value. However, by utilizing information about the character of the pain in connection with knowledge of what sensory fibres may be transmitting it, the clinician can gain valuable clues for diagnosis of the source and cause of pain, and what may therefore be necessary for treatment.
Pain of dentinal and pulpal origin can be separated into three broad groups (Fig. 3). Whilst the distinctions are of practical value, the astute clinician will be aware that these groups cover most, but not all of the various types of presenting pain.
Dentine sensitivity is a pain of a short, sharp, well-localized character which ceases immediately after removal of the stimulus. It is associated with exposed, open dentinal tubules and is mediated by A-delta fibres. This is the pain which might, for example, be experienced during cavity preparation in dentine. The pain stops when the drilling stops!
Hypersensitive dentine is a condition in which pain occurs as a result of an increased responsiveness of the tooth to stimuli which might not ordinarily induce such a response. The pain is described as sharp, and is induced by subtle thermal stimuli at what seems to be a lower threshold than normal, but the pain terminates upon removal of the stimulus. This pain is related to open dentinal tubules and an increased sensibility of pulp nerve fibres, probably as a result of a slight pulp inflammation. Such pain might, for example, occur postoperatively after root planing of teeth in which mild stimuli like cool air or beverages readily induce pain. In some patients it may be hard to distinguish clinically between dentine sensitivity and dentine hypersensitivity because of modulation of pain locally or by the central nervous system.1
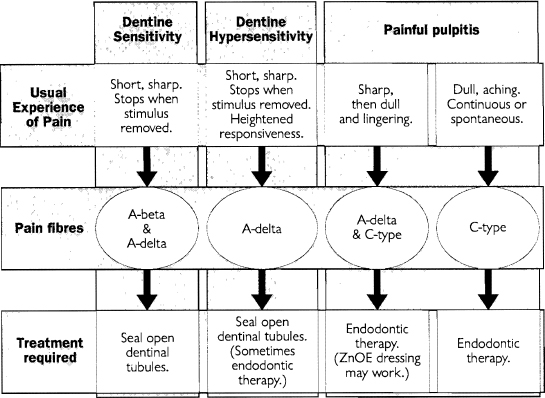
Fig. 3.—Diagram summarizing the characteristics of pain arising from the pulp, including the typical clinical features of the pain, the pain fibres responsible and the treatment most likely to be effective.
It should be understood that the term dentine hypersensitivity has been used loosely to describe what are really two different types of pain: ‘normal’ dentine sensitivity as outlined above and, as applied here, an increased responsiveness of the tooth to milder stimuli. A more accurate term than hypersensitivity is the term ‘hyperalgesia’. During inflammation, the stimulation threshold of nerve fibres can be lowered and this increased sensitivity of the nerves to stimulation is termed hyperalgesia.18,81
Painful pulpitis may present in one of two forms. One presentation occurs as a tooth which is exquisitely sensitive to hot stimuli and which responds with an instant, severe, sharp pain (indicating A-delta fibre activation) and which is then followed by a prolonged dull, sometimes radiating, aching pain (implying the involvement of C-fibres).
A more severe form of painful pulpitis is one in which there may be a diffuse dull aching pain of a throbbing, penetrating nature which is continuous or which arises spontaneously and lasts for minutes to hours. This is characteristic of C-fibre activation within the pulp.80 In many of these cases, it is likely that the dentinal tubules are open, due to caries, trauma or a leaking restoration. This not only facilitates the increased flow of fluids resulting in increased sensitivity by activating A-delta fibres, but it provides an important point of entry for micro-organisms which leads to pulp inflammation. The inflammatory mediators released into the pulp can have an excitatory or, under some circumstances, an inhibitory effect on these A-delta fibres.
Implications for diagnosis and treatment
It is imperative that the clinician takes a careful history before undertaking an examination or tests, let alone treatment. Determining the character, duration and stimuli to the pain furnishes the clinician with valuable information for both diagnosis and treatment of the pain. Some patients will describe their pain fully and coherently; others will report the barest of details stating only that they have pain. In the latter case it is then up to the clinician to determine the nature of the pain by inquiry with appropriate questions.
If the pain is of a short, sharp nature and is brought on by hydrodynamic type stimuli then it is almost certainly due to activation of A-delta fibres. This should signify to the clinician the presence of open, exposed dentinal tubules such as might occur through caries, toothbrush abrasion, cracked tooth syndrome, or trauma. It may also occur as a postoperative complication through microleakage around a restoration or as a sensitivity following scaling or periodontal surgery. The implications are then clear: the sensitive tooth must firstly be identified — the method for doing so will be addressed in a subsequent paper. Once located, the exposed tubules should be sealed. There are many agents which have been advocated for stopping dentinal sensitivity and most of them rely for their mechanism of action on their ability to seal and occlude dentinal tubules. A particularly effective agent is potassium oxalate which works well both inside cavities and on the external surface of the tooth, such as in cases of toothbrush abrasion. In most cases of dentine sensitivity (involving A-delta fibres only), the pain can be stopped and the pulp saved by these simple measures (Fig. 3).
If the patient describes symptoms of dentine hypersensitivity, the first line of treatment is initially the same as outlined above: find the source and seal the tubules. Here, however, the chances that this treatment alone will be sufficient are reduced and those cases which do not respond may require endodontic therapy.
If a patient describes the pain as one which is diffuse, dull and aching then this gives the clinician an important clue that the pain is associated with C-fibres. Since these pain fibres are usually activated when noxious damage has occurred, the clinician should expect to look for a tooth with an inflamed pulp which, in most cases, is past responding to conservative treatment. The clinician must also be alert to the possibility of referred pain since the tooth which the patient feels is the source of the pain may not be its true origin. Special care must therefore be taken to correctly identify these teeth with a painful pulpitis. Teeth which respond to stimuli with a severe, sharp pain followed by a dull ache may occasionally settle with the application of a sedative zinc oxide eugenol dressing, but in most cases, especially those with a continuous or spontaneous dull pain, endodontic therapy will be required to remove the inflamed and painful pulp.
REFERENCES
1 Sessle BJ. Recent developments in pain research: Central mechanisms of orofacial pain and its control. J Endodod 1986;12:435-44.
2 Virtanen A. Studies on the electrophysiological properties of the tooth pulp nerve fibres and experimental dental pain. Helsinki: University of Helsinki, 1991:68-70. Thesis.
3 Narhi MVO, Jyväsjärvi E, Virtanen A, Huopaniemi T, Ngassapa D, Hirvonen T. Role of intradental A- and C-type nerve fibres in dental pain mechanisms. Proc Finn Dent Soc 1992;88:Suppl 1:507-16.
4 Jyväsjärvi E, Kniffki K-D, Mengel MKC, Stiefenhofer A. Temperature-evoked sensation in human teeth: two components of pain in response to cold stimulation. In: Bligh J, Voight K, eds. Thermoreception and temperature regulation. Berlin: Springer-Verlag, 1990:116-24.
5 Jenkins GN. The physiology and biochemistry of the mouth. 4th edn. Oxford: Blackwell Scientific, 1978:562-7.
6 Lilja J. Innervation of different parts of the predentin and dentin in young human premolars. Acta Odontol Scand 1979;37:339-46.
7 Anderson DJ, Naylor MN. Chemical excitants of pain in human dentine and dental pulp. Arch Oral Biol 1962;7:413-5.
8 Brännström M. The elicitation of pain in the human dentine and pulp by chemical stimuli. Arch Oral Biol 1962;7:59-62.
9 Avery JK. A possible mechanism of pain conduction in teeth. Ann Histochem 1963;8:59-64.
10 Brännström M, Garberoglio R. The dentinal tubules and the odontoblast process. A scanning electron microscopic study. Acta Odont Scand 1972;30:291-311.
11 Maniatopoulos C, Smith DC. A scanning electron microscopic study of the odontoblast process in human coronal dentine. Arch Oral Biol 1983;28:701-10.
12 Nair PN. Innervation of root dentine in human premolars. Schweiz Monatsschr Zahmed 1993;103:965-72.
13 Brännström M, Åström A. A study on the mechanism on pain elicited from the dentin. J Dent Res 1964;43:619-25.
14 Lilja J, Nordenvall K-J, Brännström M. Dentin sensitivity, odontoblasts and nerves under desiccated or infected experimental cavities. Swed Dent J 1982;6:93-103.
15 Brännström M. Dentin and pulp in restorative dentistry. London: Wolfe Medical, 1982:9-44.
16 Brännström M. The hydrodynamic theory of dentinal pain: sensation in preparations, caries, and the dentinal crack syndrome. J Endodon 1986;12:453-7.
17 Johnson G, Olgart L, Brännström M. Outward fluid flow in dentin under a physiologic pressure gradient: experiments in vitro. Oral Surg Oral Med Oral Pathol 1973;35:238-48.
18 Trowbridge HO. Review of dental pain — histology and physiology. J Endodon 1986;12:445-52.
19 Brännström M, Åström A. The hydrodynamics of the dentine; its possible relationship to dentinal pain. Int Dent J 1972;22:219-27.
20 Seltzer S, Bender IB, Ziontz M. The dynamics of pulp inflammation: correlations between diagnostic data and actual histologic findings in the pulp. Oral Surg Oral Med Oral Pathol 1963; 16:969-77.
21 Hirvonen TJ, Närhi MVO, Hakumäki MOK. The excitability of dog pulp nerves in relation to the condition of dentine surface. J Endodon 1984;10:294-8.
22 Hirvonen TJ, Närhi MVO. The effect of dentinal stimulation on pulp nerve function and pulp morphology in the dog. J Dent Res 1986;65:1290-3.
23 Johnsen DC. Innervation of teeth: qualitative, quantitative, and developmental assessment. J Dent Res 1985;64:Spec Iss:555-63.
24 Hirvonen TJ. A quantitative electron-microscopic analysis of the axons at the apex of the canine tooth pulp in the dog. Acta Anat 1987;128:134-9.
25 Nair PNR, Luder HU, Schroeder HE. Number and size-spectra of myelinated nerve fibres of human premolars. Anat Embryol 1992;186:563-71.
26 Dörscher-Kim J, Kim S. The adrenergic system and dental pulp. In: Inoki R, Kudo T, Olgart LM, eds. Dynamic aspects of dental pulp: Molecular biology, pharmacology and pathophysiology. London: Chapman and Hall, 1990:283-96.
27 Olgart LM. Functions of peptidergic nerves. Ibid.: 349-62.
28 Närhi MVO. The characteristics of intradental sensory units and their responses to stimulation. J Dent Res 1985;64:Spec Iss:564-71.
29 Dong WK, Chudler E, Martin R. Physiological properties of intradental mechanoreceptors. Brain Res 1985;334:389-95.
30 Jyväsjärvi E. Electrophysiological studies of afferent C-fibre innervation in the dental pulp. Helsinki: University of Helsinki, 1986:87-8. Thesis.
31 Jyväsjärvi E, Kniffki K-D. Cold stimulation of teeth: a comparison between the responses of cat intradental A and C fibres and human sensation. J Physiol 1987;391:193-207.
32 Närhi MVO, Hirvonen TJ, Hakumäki MOK. Responses of intradental nerve fibres to stimulation of dentine and pulp. Acta Physiol Scand 1982;115:173-8.
33 Närhi MVO, Hirvonen TJ, Hakumäki MOK. Activation of intradental nerves in the dog to some stimuli applied to the dentine. Archs Oral Biol 1982;27:1053-8.
34 Ahlquist ML, Edwall LGA, Franzén OG» Haegerstam GAT. Perception of pulpal pain as a function of intradental nerve activity. Pain 1984;19:353-66.
35 Ngassapa D. Dentine sensitivity: factors influencing intradental nerve activation in dog teeth. Kuopio: University of Kuopio, 1991:90-2. Thesis.
36 Pashley DH. Mechanisms of dentin sensitivity. In: Curro FA, ed. Tooth hypersensitivity. Dent Clin North Am 1990;34:449-73.
37 Jyväsjärvi E, Kniffki K-D. Afferent C fibre innervation of cat tooth pulp: confirmation by electrophyiological methods. J Physiol 1989;411:663-75.
38 Närhi M, Jyväsjärvi E, Hirvonen T, Huopaniemi T. Activation of heat sensitive nerve fibres in the dental pulp of the cat. Pain 1982;14:317-26.
39 Jyväsjärvi E, Kniffki K-D, Mengel MKC. Functional characteristics of afferent C fibres from tooth pulp and periodontal ligament. In: Hamann W, Iggo A, eds. Progress in brain research. Elsevier, 1988;74:237-45.
40 Brännström M, Lindén L-Ä, Johnson G. Movement of dentinal and pulpal fluid caused by clinical procedures. J Dent Res 1968;47:679-82.
41 Jyväsjärvi E, Kniffki K-D. Comparison between human subjective ratings and response properties of cat intradental fine afferents to cold stimulation of teeth. In: Schmidt RF, Schaible H-G, VahleHinz C, eds. Fine afferent nerve fibers and pain. Weinheim: VCH Verlagsgesellschaft, 1987:117-25.
42 Eccles JD, Green RM. The conservation of teeth. Oxford: Blackwell Scientific, 1973:80.
43 Brännström M, Nyborg H. The presence of bacteria in cavities filled with silicate cement and composite resin materials. Swed Dent J 1971;64:149-55.
44 Brännström M. Communication between the oral cavity and the dental pulp associated with restorative treatment. Oper Dent 1984;9:57-68.
45 Pashley DH. Clinical considerations of microleakage. J Endodon 1990;16:70-7.
46 Cox CF. Biocompatibility of dental materials in the absence of bacterial infection. Oper Dent 1987;12:146-52.
47 Brännström M. Infection beneath composite resin restorations: can it be avoided? Oper Dent 1987;12:158-63.
48 Qvist V. Resin restorations: leakage, bacteria, pulp. Endod Dent Traumatol 1993;9:127-52.
49 Brännström M. The cause of postrestorative sensitivity and its prevention. J Endodon 1986;12:475-81.
50 Pashley DH, Galloway SE. The effects of oxalate treatment on the smear layer of ground surfaces of human dentine. Arch Oral Biol 1985;30:731-7.
51 Pashley DH, Depew DD. Effects of the smear layer, copalite, and oxalate on microleakage. Oper Dent 1986;11:95-102.
52 Brännström M, Nordenvall K-J, Torstenson B, Hedström K-G, Wåhlstam H. Protective effect of polystyrene liners for composite resin restorations. J Prosthet Dent 1983;49:331-6.
53 Torstenson B, Brännström M, Mattsson B. A new method for sealing composite resin contraction gaps in lined cavities. J Dent Res 1985;64:450-3.
54 Brännström M, Mattsson B, Torstenson B. Materials techniques for lining composite resin restorations: a critical approach. J Dent 1991;19:71-9.
55 Eick JD. Smear layer — materials surface. Proc Finn Dent Soc 1992;88:Suppl 1:225-42.
56 Pashley DH. Smear layer: physiological considerations. Oper Dent 1984;Suppl 3:13-29.
57 Pashley DH, Michelich V, Kehl T. Dentin permeability: effects of smear layer removal. J Prosthet Dent 1981;46:531-7.
58 Pashley DH, Kepler EE, Williams EC, Okabe A. The effects of acid etching on the in vivo permeability of dentine in the dog. Arch Oral Biol 1984;28:555-9.
59 Torstenson B, Brännström M. Composite resin contraction gaps measured with a fluorescent resin technique. Dent Mater 1988;4:238-42.
60 Torstenson B, Odén A. Effect of bonding agent types and incremental techniques on minimizing contraction gaps around composite resins. Dent Mater 1989;5:218-23.
61 Hirata K, Nakashima M, Sekine I, Mukouyama Y, Kimura K. Dentinal fluid movement associated with loading of restorations. J Dent Res 1991;70:975-8.
62 Pashley DH. Dentin permeability, dentin sensitivity, and treatment through tubule occlusion. J Endodon 1986;12:465-74.
63 Olgart L, Gazelius B, Brodin E, Nilsson G. Release of substance P-like immunoreactivity from the dental pulp. Acta Physiol Scand 1977;101:510-2.
64 Kim S. Neurovascular interactions in the dental pulp in health and inflammation. J Endodon 1990;16:48-53.
65 Kim S, Liu M, Simchon S, Dörscher-Kim J. Effects of selected inflammatory mediators on blood flow and vascular permeability in the dental pulp. Proc Finn Dent Soc 1982;88:Suppl 1:387-92.
66 Heyeraas KJ, Kvinnsland I. Tissue pressure and blood flow in pulpal inflammation. Proc Finn Dent Soc 1992;88:Suppl 1:393-401.
67 Edwall L, Scott D Jr. Influence of changes in microcirculation on the excitability of the sensory unit in the tooth of the cat. Acta Physiol Scand 1971;82:555-66.
68 Trowbridge HO. Intradental sensory units: physiological and clinical aspects. J Endodon 1985;11:489-98.
69 Olgart LM. The role of local factors in dentin and pulp in intradental pain mechanisms. J Dent Res 1985;64:Spec lss:572-8.
70 Olgart LM. Involvement of sensory nerves in hemodynamic reactions. Proc Finn Dent Soc 1982;88:Suppl 1:403-10.
71 Michelich VJ, Schuster GS, Pashley DH. Bacterial penetration of human dentin in vitro. J Dent Res 1980;59:1398-403.
72 Panopoulos P. Intradental sensory nerve responses to some factors affecting dentin and pulp. Stockholm: Karolinska Institute, 1983:35-6. Thesis.
73 Olgart LM. Pain research using feline teeth. J Endodon 1986;12:458-61.
74 Byers MR. Effects of inflammation on dental sensory nerves and vice versa. Proc Finn Dent Soc 1992;88:Suppl 1:499-506.
75 Gazelius B. Studies on the release and effects of putative mediators of pain in the dental pulp. Stockholm: Karolinska Institute, 1981:25-6. Thesis.
76 Ngassapa D, Närhi M, Hirvonen T. Effect of serotonin (5-HT) and calcitonin gene-related peptide (CGRP) on the function of intradental nerves in the dog. Proc Finn Dent Soc 1992;88:Suppl 1:143-8.
77 Yoshiyama M, Noiri Y, Ozaki K, Uchida A, Ishikawa Y, Ishida H. Transmission electron microscopic characterization of hypersensitive human radicular dentin. J Dent Res 1990;69:1293-7.
78 Kontturi-Närhi V. Dentine hypersensitivity — factors related to the occurrence of pain symptoms. Kuopio: University of Kuopio, 1993:100-1. Thesis.
79 Hirvonen T, Ngassapa D, Närhi M. Relation of dentin sensitivity to histological changes in dog teeth with exposed and stimulated dentin. Proc Finn Dent Soc 1988;88:Suppl 1:133-41.
80 Närhi M, Kontturi-Närhi V, Hirvonen T, Ngassapa D. Neurophysiological mechanisms of dentin hypersensitivity. Proc Finn Dent Soc 1992;88:Suppl 1:15-22.
81 Campbell JN, Raja SN, Cohen RH, Manning DC, Khan AA, Meyer RA. Peripheral neural mechanisms of nociception. In: Wall PD, Melzack R, eds. Textbook of pain. Edinburgh: Churchill Livingstone, 1989:22-45.
Medical Centre,
517 St Kilda Road,
Melbourne, Victoria 3004.
* First part of a seminar presented at the 12th Convocation, Royal Australasian College of Dental Surgeons, Canberra, April 1994.
†Endodontist, Melbourne. Part-time Lecturer and Clinical Demonstrator at the Faculty of Medicine, Dentistry and Health Sciences, University of Melbourne; Visiting Scientist at the Department of Microbiology, Faculty of Medicine, Monash University.