Mechanisms involved in the resistance of Enterococcus faecalis to calcium hydroxide
M. Evans1, J. K. Davies2, G. Sundqvist3 & D. Figdor1,2,3
1School of Dental Science, University of Melbourne, 2Department of Microbiology, Monash University, Melbourne, Australia, and 3Department of Endodontics, Umeå University, Umeå, Sweden
Abstract
Evans M, Davies JK, Sundqvist G, Figdor D. Mechanisms involved in the resistance of Enterococcus faecalis to calcium hydroxide. International Endodontic Journal, 35, 221–228, 2002.
Aim This study sought to clarify the mechanisms that enable E. faecalis to survive the high pH of calcium hydroxide.
Methodology E. faecalis strain JH2-2 was exposed to sublethal concentrations of calcium hydroxide, with and without various pretreatments. Blocking agents were added to determine the role of stress-induced protein synthesis and the cell wall-associated proton pump.
Results E. faecalis was resistant to calcium hydroxide at a pH of 11.1, but not pH 11.5. Pre-treatment with calcium hydroxide pH 10.3 induced no tolerance to further exposure at pH 11.5. No difference in cell survival was observed when protein synthesis was blocked during stress induction, however, addition of a proton pump inhibitor resulted in a dramatic reduction of cell viability of E. faecalis in calcium hydroxide.
Conclusions Survival of E. faecalis in calcium hydroxide appears to be unrelated to stress induced protein synthesis, but a functioning proton pump is critical for survival of E. faecalis at high pH.
Keywords: E. faecalis, proton pump, sodium hypochlorite, stress response.
Received 8 May 2001; accepted 24 July 2001
Introduction
Only a few studies have investigated the aetiology of failures in which the endodontic therapy has been well performed. These studies have revealed five factors that may contribute to a persistent periapical radiolucency after treatment. The factors are: intraradicular infection (Nair et al. 1990a); extraradicular infection by bacteria of the species Actinomyces israelii and Propionibacterium propionicum (Nair & Schroeder 1984, Sjögren et al. 1988); foreign body reaction (Koppang et al. 1989, Nair et al. 1990b); cysts, especially those containing cholesterol crystals (Nair et al. 1993); and fibrous scar tissue healing following conventional treatment (Nair et al. 1999). Of all these factors, it is generally believed that the major cause of root canal treatment failure is the persistence of microorganisms in the apical part of root-filled teeth.
Although information on the type of microorganisms that persist in the root-filled canal is limited, several studies have reported that root-filled teeth with persisting periapical lesions usually harbour one or a few bacterial species and that Gram-positive bacteria dominate the bacterial flora (Möller 1966, Molander et al. 1998, Sundqvist et al. 1998, Noda et al. 2000, Hancock et al. 2001). The species Enterococcus faecalis has been recovered in a high proportion of endodontic failures, approximately one third of the canals of root-filled teeth with persistent periapical lesions (Möller 1966, Molander et al. 1998, Sundqvist et al. 1998, Hancock et al. 2001).
The common recovery of E. faecalis from canals where the treatment has failed suggests it is an opportunistic pathogen whose persistence in the root canal presents a significant therapeutic problem. When E. faecalis has been identified in cases of failed endodontic treatment, it has proven to be difficult to eradicate during retreatment (Sundqvist et al. 1998), and the failure rate of retreatment is higher when E. faecalis has been recovered from the root canal at the time of root filling (Sundqvist et al. 1998). Once established in the root canal, E. faecalis faces several challenges for survival, including an ability to withstand antimicrobial agents used during treatment and endure potential starvation in the cleaned and obturated canal. E. faecalis appears to be highly resistant to the medicaments used during treatment and is known to resist the antibacterial effect of dressing with calcium hydroxide (Byström et al. 1985, Haapasalo & Ørstavik 1987, Safavi et al. 1990, Ørstavik & Haapasalo 1990, Sato et al. 1993, Weiger et al. 1995, Siqueira & de Uzeda 1996, Tanriverdi et al. 1997, Waltimo et al. 1999). Interestingly, resistance to killing by calcium hydroxide is shared with the few other microorganisms that have been associated with endodontic failure, such as Candida species (Nair et al. 1990a, Waltimo et al. 1997, Waltimo et al. 1999) and Actinomyces radicidentis (Kalfas et al. 2001).
Although E. faecalis has been reported to withstand the high pH of calcium hydroxide, relatively little is known about the survival mechanisms of E. faecalis that enable it to tolerate exposure to calcium hydroxide. In general, when bacteria face an adverse or potentially lethal challenge, a stress response is mounted that allows them to endure the threat, survive and recover (Parsell & Lindquist 1993, Welch 1993). This response has been described for a diverse range of stresses and in some bacterial species the adaptive response may contribute to pathogenesis (Foster & Hall 1990, Bearson et al. 1998, Castanie-Cornet et al. 1999). When a stress response is induced, this state may confer a general protection against a range of other stresses, for example a stress response induced by nutrient starvation provides protection against heat stress (Jouper-Jaan et al. 1992). E. faecalis has been shown to synthesize a variety of stress proteins when exposed to adverse environmental conditions (Hartke et al. 1998) including high salt concentration (Flahaut et al. 1996a), bile salts, acid, heat (Flahaut et al. 1996b), glucose starvation (Giard et al. 1997), high pH (Flahaut et al. 1997), sodium hypochlorite (Laplace et al. 1997) and starvation in tap water (Hartke et al. 1998).
If starvation or exposure to sodium hypochlorite solution or diffusing calcium hydroxide induces E. faecalis to enter a stress response then this might confer cross protection to E. faecalis when it is subsequently exposed to a calcium hydroxide dressing. This study sought to clarify the strategies employed by E. faecalis that may result in resistance to treatment. The aim was to investigate the stress response of E. faecalis to antimicrobial agents by exposing cells to sublethal concentrations of sodium hypochlorite and calcium hydroxide with or without a range of pretreatments. We also examined alternative cell mechanisms that may be important for survival of E. faecalis at high pH.
Materials and methods
Bacterial strain and culture conditions
The bacterial strain used for all experiments was E. faecalis JH2-2, which is derived from the parental strain JH2 (Jacob & Hobbs 1974) and is representative of the species. Cultures were grown overnight in 10 mL Brain Heart Infusion broth (BHI, Oxoid Ltd, Basingstoke, UK) at 37°C, in air without shaking. A 2% inoculum in 50 mL BHI was then grown at 37°C to an optical density at 600 nm (OD600) of 1.1. Preliminary experiments showed that cells stopped growing and entered stationary phase at this OD. All experiments described below were repeated in triplicate.
Antimicrobial solutions
Sodium hypochlorite solutions were prepared fresh daily by dilution of a 1% sodium hypochlorite solution (10 000 p.p.m. available chlorine, pH11; Milton®, Procter & Gamble, Parramatta, NSW, Australia) to 0.0001% or 0.005% solution (1 and 50 p.p.m. available chlorine, respectively). Available chlorine was confirmed by colorimetry (Lovibond 1000 Comparitor, Salisbury, UK).
A saturated solution of calcium hydroxide was prepared by dissolving calcium hydroxide powder (Sigma Chemical Co., St Louis, MO, USA) overnight in de-ionized water. The saturated solution was centrifuged (3000 × g, 10 min), the supernatant was removed and filtered using a Millex®-GP 0.22 µm Filter Unit (Millipore, Bedford, MA, USA) resulting in a stock calcium hydroxide solution, pH 11.5. Additional calcium hydroxide solutions of pH 11.1 and 10.3 were prepared by dilution of stock with de-ionized water (1 in 4 and 1 in 40, respectively).
Pre-treatment and challenge conditions
After cultures were grown to stationary phase, 10 mL aliquots were removed and cells were harvested by centrifugation (3000 × g, 10 min). Cells were pretreated by resuspending cell pellets in 10 mL solutions of either 0.0001% sodium hypochlorite solution or calcium hydroxide solution pH 10.3 (or de-ionized water for controls) for 30 min. One mL aliquots were placed in Eppendorf tubes and cells recovered by centrifugation (16 000 × g, 3 min) and washed before challenge.
Table 1 Effect of pretreatment on survival of E. faecalis JH2-2 challenged with sodium hypochlorite and calcium hydroxide
| Challenge | ||||||||
| NaOCl, 0.005% | Ca(OH)2, pH 11.1 | Ca(OH)2, pH 11.5 | ||||||
| Pre-treatment | 15 min | 30 min | 15 min | 30 min | 15 min | 30 min | ||
| No pretreatment | 1.2 × 10–4 | 4.4 × 10–6 | 4.8 × 10–1 | 4.1 × 10–1 | 2.6 × 10–4 | 1.3 × 10–6 | ||
| Ca(OH)2, pH 10.3 | 1.4 × 10–4 | 1.6 × 10–5 | 1.0 × 100 | 7.5 × 10–1 | 5.7 × 10–4 | 6.7 × 10–6 | ||
| NaOCl, 0.0001% | 8.1 × 10–5 | 4.3 × 10–6 | 2.3 × 10–1 | 1.3 × 10–1 | 1.8 × 10–4 | 1.7 × 10–6 | ||
Data expressed as percentage survival, average of 3 experiments.
Number of cells at 0 min was 2.3 ± 0.2 × 109 (= 100%).
Bacteria were challenged by exposure to sodium hypochlorite (0.005%), or calcium hydroxide solution (pH 11.1 or 11.5). At 0, 15 and 30 min, 1 mL samples were removed, cells recovered by centrifugation (16 000 × g, 3 min), washed, and resuspended in 0.9% sodium chloride solution. Viable counts were determined by serial dilution and plating onto BHI agar. The number of colony forming units (CFU) was determined after incubation for 48 h at 37°C in air.
Mechanisms of survival-response to lethal treatment
To check if protein synthesis was necessary for cell survival, some experiments were repeated in the presence of a protein synthesis inhibitor, chloramphenicol (100 µg mL–1), which was added to the pretreatment and challenge solutions in relevant experiments. Chloramphenicol at a concentration of 100 µg mL–1 has been shown to block protein synthesis (Giard et al. 1996, Giard et al. 1997).
To determine if a proton pump was involved in resistance to high pH, nonadapted cell pellets were exposed to 1 mL calcium hydroxide (pH 11.1) in the presence of a proton pump inhibitor, carbonyl cyanide m-chlorophenylhydrazone (CCCP, 100 µM) (ICN Biomedicals Inc., Aurora, OH, USA), for 15 and 30 min. CCCP has been shown to block the normal function of the proton pump at this concentration (Mugikura et al. 1990). Control experiments were performed under identical conditions, but with the calcium hydroxide solution adjusted to pH 7.0 by dilution. Cells were recovered and viability determined as above.
Results
Response to sodium hypochlorite
Preliminary experiments revealed that sodium hypochlorite solution at a 0.5 and 1% (v/v) concentration was rapidly lethal, but E. faecalis survived a 0.0001% sodium hypochlorite solution (data not shown). Therefore, a sublethal concentration of 0.005% (v/v) sodium hypochlorite was used, which resulted in just 1.2 × 10–4% of cells surviving after 15 min and 4.4 × 10–6% cell survival after 30 min exposure (Table 1). Pre-treatment with 0.0001% sodium hypochlorite or calcium hydroxide pH 10.3 for 30 min did not induce tolerance or increase survival of E. faecalis in 0.005% sodium hypochlorite (Table 1).
Response to calcium hydroxide
When cells were exposed to calcium hydroxide pH 11.1 for 30 min, 0.4% cells survived, but a relatively small increase in alkalinity to pH 11.5 resulted in a dramatic drop in survival to just 1.3 × 10–6% (Table 1). Pretreatment with 0.0001% sodium hypochlorite had no effect on survival of cells challenged with calcium hydroxide pH 11.1 or 11.5 (Table 1). Pre-treatment with calcium hydroxide pH 10.3 for 30 min conferred no resistance by E. faecalis to further exposure to calcium hydroxide.
Effect of blocking protein synthesis
The protein synthesis inhibitor, chloramphenicol, was used to block protein production during antimicrobial challenge to determine the role of stress-induced proteins in cell survival. In the presence of 100 µg mL–1 chloramphenicol, there was no discernible difference in cell survival when cells were exposed to 0.005% sodium hypochlorite, with or without calcium hydroxide pH 10.3 pretreatment (Table 2). Similarly, there was no difference in cell survival when cells were exposed to calcium hydroxide (pH 11.1 and 11.5) in the presence of chloramphenicol, whether or not they were pretreated with calcium hydroxide pH 10.3 (Table 2).
Table 2 Effect of blocking protein synthesis with Chloramphenicol (Cn) on survival of E. faecalis JH2-2 when challenged with sodium hypochlorite and calcium hydroxide, with and without pretreatment
| Challenge | ||||||||
| NaOCl, 0.005% | Ca(OH)2, pH 11.1 | Ca(OH)2, pH 11.5 | ||||||
| Pre-treatment | 15 min | 30 min | 15 min | 30 min | 15 min | 30 min | ||
| No pretreatment* | 1.2 × 10–4 | 4.4 × 10–6 | 4.8 × 10–1 | 4.1 × 10–1 | 2.6 × 10–4 | 1.3 × 10–6 | ||
| No pretreatment | ||||||||
| + Cn | 1.1 × 10–4 | 7.8 × 10–6 | 4.9 × 10–1 | 3.7 × 10–1 | 2.6 × 10–4 | 1.6 × 10–6 | ||
| Ca(OH)2, pH 10.3* | 1.4 × 10–4 | 1.6 × 10–5 | 1.0 × 100 | 7.5 × 10–1 | 5.7 × 10–4 | 6.7 × 10–6 | ||
| Ca(OH)2, pH 10.3 + Cn | 1.1 × 10–4 | 9.0 × 10–6 | 9.7 × 10–1 | 7.5 × 10–1 | 7.4 × 10–4 | 6.8 × 10–6 | ||
Data expressed as percentage survival, average of three experiments.
Number of cells at 0 min was 2.3 ± 0.2 × 109 (= 100%).
*Data from Table 1 included for comparison.
Effect of blocking proton pump
When the environmental pH rises, bacteria attempt to preserve the intracellular pH by pumping protons across the cytoplasmic membrane to maintain the cytoplasmic pH (Booth 1985, Booth 1999). The proton pump inhibitor, CCCP, was used to determine whether this mechanism might play a role in tolerance of E. faecalis to alkaline conditions.
When cells were exposed to calcium hydroxide at pH 11.1 for 30 min, there was a 20-fold reduction in cell survival in the presence of CCCP compared to calcium hydroxide exposure in the absence of the proton pump inhibitor (Fig. 1). The effect of CCCP was apparently time dependent because the effect was even more pronounced after 60 min of calcium hydroxide exposure (one experiment only; without CCCP 6.4 × 10–3% of cells survived, but in the presence of CCCP, survival was 9.0 × 10–5%). Control experiments showed that CCCP was not lethal to E. faecalis at pH 7.0 (Fig. 1).
Discussion
The common recovery of E. faecalis in root canals of teeth where endodontic treatment has failed implies that this species is intimately involved in the pathogenesis and maintenance of persisting apical periodontitis. One feature that may enable E. faecalis to persist in the root canal is an ability for it to survive conventional antimicrobial agents used during endodontic treatment, such as the alkaline pH of calcium hydroxide. E. faecalis is known to withstand a high pH; indeed this is an identifying characteristic of E. faecalis (Mundt 1986, Devriese et al. 1992). At pH 11.5 or greater, E. faecalis does not survive, yet it can survive at a pH below 11.5 as shown in this and earlier studies (Byström et al. 1985). Because of the buffering effect of dentine (Wang & Hume 1988, Nerwich et al. 1993, Haapasalo et al. 2000), it is unlikely that the high pH of calcium hydroxide (>11.5) is attained within dentinal tubules where E. faecalis has the capacity, at least in vitro, to penetrate deeply (Haapasalo & Ørstavik 1987, Ørstavik & Haapasalo 1990, Safavi et al. 1990, Vahdaty et al. 1993, Peters et al. 2000). In radicular dentine, alkalinity may only reach pH 10.3 after dressing the canal with calcium hydroxide (Nerwich et al. 1993, Miñana et al. 2001).
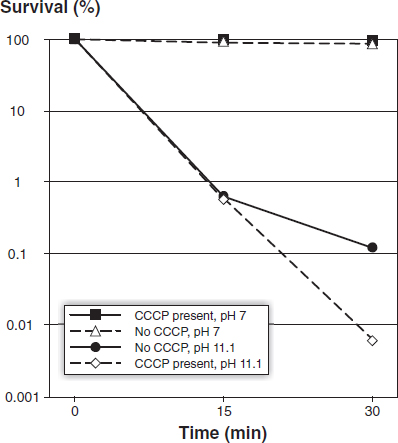
Figure 1 Survival of E. faecalis JH2-2 in calcium hydroxide pH 11.1 (and controls, pH 7.0) in the presence or absence of CCCP. Each data point is the average of 3 experiments.
Because endodontic treatment involves repeated or alternate use of sodium hypochlorite solution and calcium hydroxide at various stages of treatment, E. faecalis is likely to be exposed to an alkaline pH, which is sublethal. These conditions could adapt or ‘pretreat’ cells by eliciting a stress response that enhances their survival. For example, adaptation of E. faecalis by incubation at low temperatures confers an increased ability for cells to withstand freezing (Thammavongs et al. 1996). It therefore seems reasonable to suspect that repeated exposure of E. faecalis to sodium hypochlorite solution and calcium hydroxide might induce resistance to subsequent exposure at levels that might normally be lethal.
The initial experiments confirmed the survival of E. faecalis in an alkaline high pH. After 30 min exposure in calcium hydroxide pH 11.1, some cells (0.4%) survived, however, at pH 11.5 >99.99% were killed. Next, E. faecalis was pretreated with calcium hydroxide pH 10.3 before challenging cells in calcium hydroxide (pH 11.1 and 11.5) and these experiments revealed a minimal increase in survival by E. faecalis, much less than reported in another study (Flahaut et al. 1997). Pre-treatment with sodium hypochlorite solution had no effect on survival by E. faecalis in calcium hydroxide. Thus, these results show that pretreatment by calcium hydroxide and sodium hypochlorite solution is of minor importance in the ability of E. faecalis to survive the high alkalinity of calcium hydroxide.
The capacity to mount a stress response with synthesis of general or specific stress-induced proteins is an important survival mechanism (Foster & Spector 1995). The production of these proteins is a fundamental biological response in many bacterial species and protects cells from adverse conditions, facilitating cell recovery when more favourable conditions are re-established (Parsell & Lindquist 1993, Welch 1993). E. faecalis has been shown to produce a number of such proteins in response to other environmental stresses, including glucose starvation (Giard et al. 1997), elevated temperatures (Boutibonnes et al. 1993) and bile salts (Flahaut et al. 1996b). To determine the role of stress-induced proteins in surviving the alkaline pH of calcium hydroxide, protein production was blocked with chloramphenicol during pretreatment and challenge with calcium hydroxide. Stopping protein synthesis had no effect on cell survival, which implies that stress-induced protein production is not important for survival of E. faecalis at high pH, a finding consistent with Flahaut et al. (1997). The induction of tolerance to high pH was independent of protein synthesis because pre-treatment in the presence of chloramphenicol did not alter cell survival.
In acid or alkaline environments, most bacterial cells maintain pH homeostasis in which the internal pH is kept within a narrow range so that enzymes and proteins maintain normal function (Booth 1985). The pH homeostasis is based on two principal components: a passive function consisting of a low cell membrane permeability to ions and a buffering ability of the cytoplasm; and an active mechanism that functions mainly through controlled transport of cations (potassium, sodium and protons) across the cell membrane (Booth 1985, Kakinuma 1998, Booth 1999). In acidic environments, a cation antiport system is thought to raise the internal pH by expelling protons across the cell wall. In an alkaline medium, cations/protons are pumped into the cell to lower the internal pH (Booth 1985, Hall et al. 1995, Booth 1999). Extensive work with Enterococcus hirae (formerly Streptococcus faecalis) has confirmed a fundamental role for a potassium/proton antiport system in maintaining cytoplasmic pH in an alkaline environment (Kakinuma 1987, Kakinuma & Igarashi 1990, Kakinuma & Igarashi 1995, Kakinuma & Igarashi 1999).
In order to determine the role of the proton pump in maintaining survival of E. faecalis in a high pH environment, CCCP was used to shut down the pump. CCCP is known to block proton movement across cell membranes (Kinoshita et al. 1984). In the presence of CCCP, there was a 20-fold reduction in cell survival after 30 min exposure to high pH compared to cells that were not exposed to CCCP. The effect of CCCP was apparently time-dependent because the effect was more pronounced – a 70-fold reduction – after 60 min of calcium hydroxide exposure (one experiment only). These results show that a functioning proton pump, which drives protons into the cell to acidify the cytoplasm, is critical for the survival of E. faecalis in a highly alkaline environment. Presumably, when the environmental alkalinity reaches pH 11.5 or above, this life-saving mechanism is overwhelmed.
During clinical endodontic treatment, sodium hypochlorite solution is usually used in a concentration range of 0.5–5% and preliminary experiments confirmed that exposure to 0.5 and 1% sodium hypochlorite solution for 5 min was lethal to E. faecalis (data not shown). Indeed, exposure to 0.005% sodium hypochlorite resulted in >99.99% killing of E. faecalis at 30 min. It should be noted that these concentrations are considerably below those used in conventional clinical treatment, yet even at this low concentration sodium hypochlorite solution was highly bactericidal. Pre-treatment with 0.0001% sodium hypochlorite did not induce tolerance against further exposure to sodium hypochlorite, which is consistent with another study (Laplace et al. 1997) showing that pretreatment of E. faecalis with sodium hypochlorite had no effect on cell survival to further sodium hypochlorite challenge. In all probability, any stress or adaptive response mounted by E. faecalis is completely overwhelmed by the lethal effect of sodium hypochlorite.
Several in vitro studies have shown sodium hypochlorite solution to be lethal to E. faecalis, yet when E. faecalis-infected dentine is exposed to sodium hypochlorite solution, some cells of E. faecalis may survive (Ørstavik & Haapasalo 1990, Vahdaty et al. 1993, Siqueira et al. 1997, Heling & Chandler 1998). The recovery of E. faecalis in the root canals of failed cases after endodontic retreatment (Sundqvist et al. 1998) also implies an ability of E. faecalis to survive, albeit rarely, the antimicrobial action of sodium hypochlorite in the root canal. Our findings show that an adaptive stress response to sodium hypochlorite solution does not account for survival of E. faecalis. Another possible reason could be strain variation – some strains of E. faecalis have been reported to survive low concentrations of sodium hypochlorite (Kearns et al. 1995). However, more likely explanations include the possibility that sodium hypochlorite solution occasionally may not reach places where E. faecalis may hide, such as in the dentinal tubules or the more inaccessible areas of the root canal system; or, that sodium hypochlorite is buffered by dentine (Haapasalo et al. 2000).
In summary, this study has confirmed that E. faecalis is resistant to killing by calcium hydroxide at or below pH 11.1. An adaptive response in alkaline pH and stress-induced protein synthesis appear to play minor roles in cell survival, however, a functioning proton pump with the capacity to acidify the cytoplasm is critical for survival of E. faecalis at high pH. E. faecalis is efficiently killed by sodium hypochlorite; in fact, the concentrations used during clinical treatment are many orders of magnitude higher than that required for lethal action. These findings contribute to our understanding of the response by E. faecalis to these antimicrobial agents, yet eradication of E. faecalis from an infected root canal remains a significant clinical challenge for modern endodontic treatment.
Acknowledgements
This study was supported by grants from the Australian Society of Endodontology Inc, the Australian Society of Endodontology (Vic Branch), the Australian Dental Research Foundation Inc and Melbourne University.
References
Bearson B, Wilson L, Foster J (1998) A low pH-inducible, PhoPQ-dependent acid tolerance response protects Salmonella typhimurium against inorganic acid stress. Journal of Bacteriology 180, 2409–17.
Booth I (1985) Regulation of cytoplasmic pH in bacteria. Microbiological Reviews 49, 359–78.
Booth I (1999) The regulation of intracellular pH in bacteria. Novartis Foundation Symposium 221, 19–37.
Boutibonnes P, Giard J, Hartke A, Thammavongs B, Auffray Y (1993) Characterization of the heat shock response in Enterococcus faecalis. Antonie Van Leeuwenhoek 64, 47–55.
Byström A, Claesson R, Sundqvist G (1985) The antibacterial effect of camphorated paramonochlorophenol, camphorated phenol and calcium hydroxide in the treatment of infected root canals. Endodontics and Dental Traumatology 1, 170–5.
Castanie-Cornet M-P, Penfound T, Smith D, Elliott J, Foster J (1999) Control of acid resistance in Escherichia coli. Journal of Bacteriology 181, 3525–35.
Devriese L, Collins M, Wirth R (1992) The Genus Enterococcus. In: The Prokaryotes. A Handbook on the Biology of Bacteria: Ecophysiology, Isolation, Identification, Applications, 2nd edn, pp. 1465–81. New York, USA: Springer-Verlag.
Flahaut S, Benachour A, Giard J-C, Boutibonnes P, Auffray Y (1996a) Defence against lethal treatments and de novo protein synthesis induced by NaCl in Enterococcus faecalis ATCC 19433. Archives of Microbiology 165, 317–24.
Flahaut S, Hartke A, Giard J-C, Auffray Y (1997) Alkaline stress response in Enterococcus faecalis: Adaptation, cross-protection, and changes in protein synthesis. Applied and Environmental Microbiology 63, 812–4.
Flahaut S, Hartke A, Giard J-C, Benachour A, Boutibonnes P, Auffray Y (1996b) Relationship between stress response towards bile salts, acid and heat treatment in Enterococcus faecalis. FEMS Microbiology Letters 138, 49–54.
Foster J, Hall H (1990) Adaptive acidification tolerance response of Salmonella typhimurium. Journal of Bacteriology 172, 771–8.
Foster J, Spector M (1995) How Salmonella survive against the odds. Annual Review of Microbiology 49, 145–74.
Giard J-C, Hartke A, Flahaut S, Benachour A, Boutibonnes P (1996) Starvation-induced multiresistance in Enterococcus faecalis JH2-2. Current Microbiology 32, 264–71.
Giard J-C, Hartke A, Flahaut S, Boutibonnes P, Auffray Y (1997) Glucose starvation response in Enterococcus faecalis JH2-2: survival and protein analysis. Research in Microbiology 148, 27–35.
Haapasalo M, Ørstavik D (1987) In vitro infection and disinfection of dentinal tubules. Journal of Dental Research 66, 1375– 9.
Haapasalo H, Sirén E, Waltimo T, Ørstavik D, Haapasalo M (2000) Inactivation of local root canal medicaments by dentine: an in vitro study. International Endodontic Journal 33, 126–31.
Hall H, Karem K, Foster J (1995) Molecular responses of microbes to environmental pH stress. Advances in Microbial Physiology 37, 229–72.
Hancock HH, Sigurdsson A, Trope M, Moiseiwitsch J (2001) Bacteria isolated after unsuccessful endodontic treatment in a North American population. Oral Surgery Oral Medicine Oral Pathology, Oral Radiology and Endodontics 91, 579–86.
Hartke A, Giard J-C, Laplace J-M, Auffray Y (1998) Survival of Enterococcus faecalis in an oligotrophic microcosm: Changes in morphology, development of general stress resistance, and analysis of protein synthesis. Applied and Environmental Microbiology 64, 4238–45.
Heling I, Chandler N (1998) Antimicrobial effect of irrigant combinations within dentinal tubules. International Endodontic Journal 31, 8–14.
Jacob A, Hobbs S (1974) Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. Journal of Bacteriology 117, 360–72.
Jouper-Jaan Å, Goodman A, Kjelleberg S (1992) Bacteria starved for prolonged periods develop increased protection against lethal temperatures. FEMS Microbiology Ecology 101, 229–36.
Kakinuma Y (1987) Lowering of cytoplasmic pH is essential for growth of Streptococcus faecalis at high pH. Journal of Bacteriology 169, 4403–5.
Kakinuma Y (1998) Inorganic cation transport and energy transduction in Enterococcus hirae and other streptococci. Microbiology and Molecular Biology Reviews 62, 1021–45.
Kakinuma Y, Igarashi K (1990) Mutants of Streptococcus faecalis sensitive to alkaline pH lack Na+-ATPase. Journal of Bacteriology 172, 1732–5.
Kakinuma Y, Igarashi K (1995) Potassium/proton antiport system of growing Enterococcus hirae at high pH. Journal of Bacteriology 177, 2227–9.
Kakinuma Y, Igarashi K (1999) Isolation and properties of Enterococcus hirae mutants defective in the potassium/proton antiport system. Journal of Bacteriology 181, 4103–5.
Kalfas S, Figdor D, Sundqvist G (2001) A new bacterial species associated with failed endodontic treatment: Identification and description of Actinomyces radicidentis. Oral Surgery Oral Medicine Oral Pathology, Oral Radiology and Endodontics 92, 208–14.
Kearns A, Freeman R, Lightfoot N (1995) Nosocomial enterococci: resistance to heat and sodium hypochlorite. Journal of Hospital Infection 30, 193–9.
Kinoshita N, Unemoto T, Kobayashi H (1984) Proton motive force is not obligatory for growth of Escherichia coli. Journal of Bacteriology 160, 1074–7.
Koppang H, Koppang R, Solheim T, Aarnes H, Stölen S (1989) Cellulose fibers from endodontic paper points as an etiological factor in postendodontic periapical granulomas and cysts. Journal of Endodontics 15, 369–72.
Laplace J-M, Thuault M, Hartke A, Boutibonnes P, Auffray Y (1997) Sodium hypochlorite stress in Enterococcus faecalis: Influence of antecedent growth conditions and induced proteins. Current Microbiology 34, 284–9.
Miñana M, Carnes D, Walker W III (2001) pH changes at the surface of root dentin after intracanal dressing with calcium oxide and calcium hydroxide. Journal of Endodontics 27, 43 –5.
Molander A, Reit C, Dahlén G, Kvist T (1998) Microbiological status of root-filled teeth with apical periodontitis. International Endodontic Journal 31, 1–7.
Möller Å (1966) Microbiological examination of root canals and periapical tissues of human teeth. Odontologisk Tidskrift (special issue) 74, 1–380.
Mugikura S, Nishikawa M, Igarashi K, Kobayashi H (1990) Maintenance of a neutral cytoplasmic pH is not obligatory for growth of Escherichia coli and Streptococcus faecalis at an alkaline pH. Journal of Biochemistry 108, 86–91.
Mundt J (1986) Enterococci. In: Bergey’s Manual of Systematic Bacteriology, Vol. 2, pp. 1063–5. Baltimore, USA: Williams & Wilkins.
Nair P, Schroeder H (1984) Periapical actinomycosis. Journal of Endodontics 10, 567–70.
Nair P, Sjögren U, Figdor D, Sundqvist G (1999) Persistent periapical radiolucencies of root-filled human teeth, failed endodontic treatments, and periapical scars. Oral Surgery Oral Medicine Oral Pathology, Oral Radiology and Endodontics 87, 617–27.
Nair P, Sjögren U, Krey G, Kahnberg K-E, Sundqvist G (1990a) Intraradicular bacteria and fungi in root-filled, asymptomatic human teeth with therapy-resistant periapical lesions: a long-term light and electron microscopic follow-up study. Journal of Endodontics 16, 580–8.
Nair P, Sjögren U, Krey G, Sundqvist G (1990b) Therapy-resistant foreign body giant cell granuloma at the periapex of a root-filled human tooth. Journal of Endodontics 16, 589–95.
Nair P, Sjögren U, Schumacher E, Sundqvist G (1993) Radicular cyst affecting a root-filled human tooth: a long-term post-treatment follow-up. International Endodontic Journal 26, 225–33.
Nerwich A, Figdor D, Messer H (1993) pH changes in root dentin over a 4-week period following root canal dressing with calcium hydroxide. Journal of Endodontics 19, 302–6.
Noda M, Komatsu H, Inoue S, Sano H (2000) Antibiotic susceptibility of bacteria detected from the root canal exudate of persistent apical periodontitis. Journal of Endodontics 26, 221–4.
Ørstavik D, Haapasalo M (1990) Disinfection by endodontic irrigants and dressings of experimentally infected dentinal tubules. Endodontics and Dental Traumatology 6, 142–9.
Parsell D, Lindquist S (1993) The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annual Review of Genetics 27, 437–96.
Peters L, Wesselink P, Moorer W (2000) Penetration of bacteria in bovine root dentine in vitro. International Endodontic Journal 33, 28–36.
Safavi K, Spångberg L, Langeland K (1990) Root canal dentinal tubule disinfection. Journal of Endodontics 16, 207–10.
Sato T, Hoshino E, Uematsu H, Noda T (1993) Predominant obligate anaerobes in necrotic pulps of human deciduous teeth. Microbial Ecology in Health and Disease 6, 269–75.
Siqueira J, de Uzeda M (1996) Disinfection by calcium hydroxide pastes of dentinal tubules infected with two obligate and one facultative anaerobic bacteria. Journal of Endodontics 22, 674–6.
Siqueira J, Machado A, Silveira R, Lopes H, de Uzeda M (1997) Evaluation of the effectiveness of sodium hypochlorite used with three irrigation methods in the elimination of Enterococcus faecalis from the root canal, in vitro. International Endodontic Journal 30, 279–82.
Sjögren U, Happonen R, Kahnberg K, Sundqvist G (1988) Survival of Arachnia propionica in periapical tissue. International Endodontic Journal 21, 277–82.
Sundqvist G, Figdor D, Persson S, Sjögren U (1998) Micro-biologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surgery Oral Medicine Oral Pathology, Oral Radiology and Endodontics 85, 86–93.
Tanriverdi F, Esener T, Erganii, Belli S (1997) An in vitro test model for investigation of disinfection of dentinal tubules infected with Enterococcus faecalis. Brazilian Dental Journal 8, 67–72.
Thammavongs B, Corroler D, Panoff J-M, Auffray Y, Bouttibonnes P (1996) Physiological response of Enterococcus faecalis JH2-2 to cold shock: growth at low temperatures and freezing/thawing challenge. Letters in Applied Microbiology 23, 398–402.
Vahdaty A, Pitt Ford T, Wilson R (1993) Efficacy of chlorhexidine in disinfecting dentinal tubules in vitro. Endodontics and Dental Traumatology 9, 243–8.
Waltimo T, Sirén E, Ørstavik D, Haapasalo M (1999) Susceptibility of oral Candida species to calcium hydroxide in vitro. International Endodontic Journal 32, 94–8.
Waltimo T, Sirén E, Torkko HL, Olsen I, Haapasalo MP (1997) Fungi in therapy-resistant apical periodontitis. International Endodontic Journal 30, 96–101.
Wang J, Hume W (1988) Diffusion of hydrogen ion and hydroxyl ion from various sources through dentin. International Endodontic Journal 21, 17–26.
Weiger R, Manncke B, Werner H, Löst C (1995) Microbial flora of sinus tracts and root canals of non-vital teeth. Endodontics and Dental Traumatology 11, 15–9.
Welch W (1993) How cells respond to stress. Scientific American 268, 56–64.
Correspondence: Dr David Figdor, 517 St Kilda Road, Melbourne, VIC 3004 Australia (tel: + 61 39866 4528; fax: + 61 39820 3102; e-mail: [email protected]