Starvation response and growth in serum of Fusobacterium nucleatum, Peptostreptococcus anaerobius, Prevotella intermedia, and Pseudoramibacter alactolyticus
Malin Brundin, DDS,a David Figdor, MDSc, FRACDS, DipEndo, PhD, FASM,b Göran Sundqvist, DDS, PhD,a and Ulf Sjögren, DDS, PhD,a Umeå, Sweden; and Melbourne, Australia
UMEÅ UNIVERSITY AND MONASH UNIVERSITY
The microbiota inhabiting the untreated root canal differ markedly from those found in post-treatment disease, yet there is limited information on the microbial characteristics distinguishing the different infections. We hypothesized that starvation survival is a key microbial property in species selection. This study analyzed starvation-survival behavior over 60 days of species representative of the untreated root canal infection: Fusobacterium nucleatum, Peptostreptococcus anaerobius, Prevotella intermedia and Pseudoramibacter alactolyticus. All species did not survive 1 day in water. In 1% serum, the 4 species could not survive beyond 2-3 weeks. They required a high initial cell density and ≥10% serum to survive the observation period. The results highlight a poor starvation-survival capacity of these 4 species compared with species prevalent in post-treatment infection, which are well equipped to endure starvation and survive in low numbers on minimal serum. These findings point to starvation-survival capacity as a selection factor for microbial participation in post-treatment disease. (Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009;108:129-134)
Availability of suitable nutrition is a prime environmental factor regulating selection, establishment and survival of microorganisms. In the infected pulp space, nutrients that sustain the microbial community may be derived from the mouth, degrading connective tissue,1 other bacteria,1-3 or periapical tissue fluid. Therefore, in the early phase of root canal infection facultative anaerobes that use carbohydrates from the oral cavity will prevail, and in the later stages of infection obligate anaerobic bacteria dominate because they can use tissue remnants and serum proteins as a nutritional supply.4-6
During endodontic treatment, necrotic debris and infection are progressively eliminated from the root canal space, which leads to a drastic change in the root canal environment. Tissue remnants are removed, nutritional interrelationships in microbial biofilms are disrupted,7 irrigants and medicaments destroy bacteria, and the supply of nutrients from the oral cavity is shut down. When the root canal is obturated, any cells fortunate to survive the antimicrobial treatment in scant pockets within the root canal space do so in small sessile biofilms, aggregates, or planktonic forms8-10 deprived of nutrients necessary for normal growth.
An ability to survive extended periods of nutrient deprivation may be important for pathogenesis in microbes that persist in the previously obturated root canal system. We have previously shown that Enterococcus faecalis, a species frequently isolated from the root canals of root-filled teeth with persistent periapical lesions,11-15 is capable of withstanding prolonged periods of starvation in a minimal metabolic state.16
Another species identified in post-treatment apical periodontitis but seldom in untreated root canal infections, Candida albicans,8,11-15,17,18 has also been shown to survive starvation for extended periods (Richards et al, manuscript in preparation). Although E. faecalis and C. albicans are representative of species in persistent root canal infections that survive starvation16 (Richards et al, manuscript in preparation), it is hitherto unknown whether this is a distinguishing characteristic separating these species from those that are usually present in the initial root canal infection. The species Fusobacterium nucleatum, Peptostreptococcus anaerobius, Prevotella intermedia, and Pseudoramibacter alactolyticus (formerly Eubacterium alactolyticum) are typical of the species that form a polymicrobial consortium prevalent in untreated root canal infections.19,20 In culture-based studies, they are rarely recovered from root canals of failed cases and if so, not as single species.11,12,18,21 Using molecular analysis, some studies have reported a higher prevalence of sequence-specific DNA from these species, but rarely as the sole species.22,23
We hypothesized that starvation survival is a key microbial attribute involved in selection of species that participate in post-treatment disease. By comparing starvation survival behaviour of species prevalent in pre- and post-treatment infection, the role of this property can be clarified as a distinguishing trait. Specifically, the aim of the present study was to evaluate the starvation-survival characteristics of the anaerobic species F. nucleatum, P. anaerobius, P. intermedia and P. alactolyticus and compare this with previously derived data on starvation-survival behavior from the persistent pathogens C. albicans and E. faecalis.
MATERIALS AND METHODS
Bacterial strains and culture conditions
Peptostreptococcus anaerobius strain C11b-a, Prevotella intermedia strain AB13a-f, Fusobacterium nucleatum strain UJA11-a, and Pseudoramibacter alactolyticus strain AB13a-n19 were used in these experiments. The bacteria were grown at 37°C for 3-4 days on blood agar in an anaerobic box in an atmosphere of 10% H2 and 5% CO2 in nitrogen. Fastidious Anaerobe agar (FAA; Laboratory M, Bury, U.K.) with the addition of 10 mg/L vitamin K was used in plate preparation. The cells were harvested from plates and resuspended in phosphate-buffered saline (PBS) or water.
Sera from 4 healthy human adults were pooled (PHS), inactivated at 56°C for 30 min, and stored at–20°C until used. Distilled filtered sterile water, PBS, and sera were prereduced for >24 h in the anaerobic box before use in the experiments.
Starvation of cells
Starvation cultures were prepared by inoculating cells at densities of 103-108 colony-forming units (cfu)/mL in water, PBS, and PHS (1%, 10%, and 50% diluted in PBS) for up to 8 weeks. All cultures were maintained in the anaerobic box throughout the starvation period. At predetermined time intervals, 100-µL aliquots were withdrawn, and starvation-survival was determined by viable counts of serial dilutions in PBS and plating on blood agar.
RESULTS
The kinetics of starvation survival of the species in 1%, 10% and 50% PHS and PBS are presented in Fig. 1. All species were tested with a range of cell densities, and representative experiments are shown for the lowest starting cell density at which the cells survived for >7 weeks in 10% and 50% PHS and survival in PBS and 1% PHS at similar cell densities (Fig. 1). Survival was dependent on serum concentration and starting cell density. In water, none of the 4 species survived for 1 day, even when the initial cell density was >108 cfu/mL (data not shown).
Fusobacterium nucleatum survived in 10% and 50% PHS for >8 weeks when the initial cell density was >106 cfu/mL (Fig. 1, A). There was a gradual decline in cell density, and after 8 weeks the density was approximately 104 cfu/mL. In 1% PHS and PBS, a dramatic drop in cell survival occurred and no cells were recovered after 3 and 7 days, respectively.
Peptostreptococcus anaerobius survived for 8 weeks in 10% and 50% PHS when the cell density was >106 cfu/mL (Fig. 1, B). After an adaptation period (3 weeks) the density rose to 104 cfu/mL. At similar initial starting densities, in PBS and 1% PHS, no cells were recovered at 2 and 3 weeks, respectively.
Pseudoramibacter alactolyticus survived in 10% and 50% PHS for 8 weeks when the starting cell density was >107 cfu/mL (Fig. 1, C). There was a gradual decline in cell density during the first 3 weeks, and after a slight recovery the cell density reached ~103 cfu/mL. In PBS and 1% PHS, at the same starting cell density, P. alactolyticus was not recoverable at 1 and 2 weeks, respectively.
In 10% and 50% PHS, P. intermedia survived for 7 weeks when the starting cell density was >107 cfu/mL (Fig. 1, D). The survival kinetics fluctuated, eventually dropping to approximately 104 cfu/mL. At the same starting cell density in PBS and 1% PHS, P. intermedia could not be recovered at 1 week.
In summary, all species required at least 10% PHS to survive the whole observation period. When starved in water, survival was <1 day and in nutrient limited conditions, all 4 species could not survive beyond 2-3 weeks. Survival was no better at higher initial concentrations (data not shown).
DISCUSSION
Only a limited number of species have been isolated in post-treatment root canal infections, which points to a strong selection pressure favoring microorganisms with particular characteristics. We hypothesized that an ability to endure starvation and use serum are important properties for species to survive root filling and participate in post-treatment infection. In previous studies (Richards et al, manuscript in preparation),16 E. faecalis and C. albicans were shown to possess a capacity to endure extended periods of starvation, yet there has been no investigation of the starvation-survival behavior of anaerobes typically found in untreated root canal infections. Therefore, we analyzed 2 gram-negative (F. nucleatum and P. intermedia) and 2 gram-positive (P. anaerobius and P. alactolyticus) obligate anaerobes and found similar survival patterns: an inability to survive starvation in water for 24 h or to survive nutrient limitation in PBS and 1% serum for 1–3 weeks, even with a high starting cell density (>107 cfu/mL). These results highlight a survival pattern distinctly different from E. faecalis and C. albicans, which both exhibit a strong capacity for starvation survival and growth in low concentrations of serum from low starting cell densities.
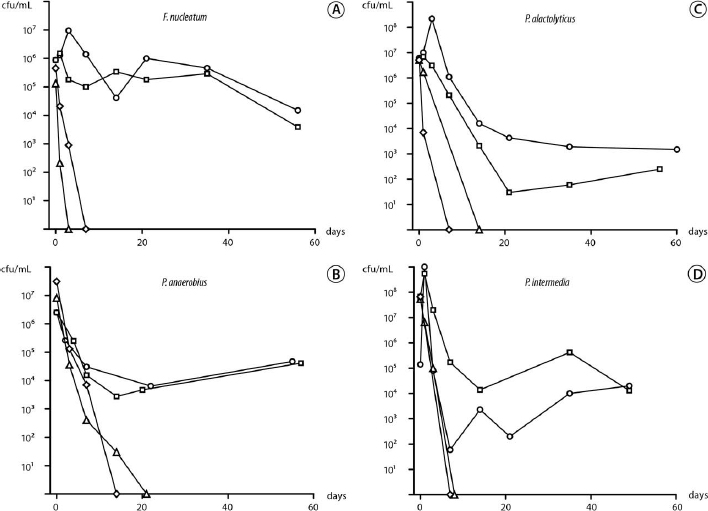
Fig. 1. Kinetics of serum-rich, nutrient-limited, and starvation survival over 60 days in serum at 50% (circles), 10% (squares), and 1% (triangles), and phosphate-buffered saline (diamonds) for the 4 species: Fusobacterium nucleatum (A), Peptostreptococcus anaerobius (B), Pseudoramibacter alactolyticus (C) and Prevotella intermedia (D).
Despite considerable interest in the role, mechanisms and molecular regulation of starvation survival in many microorganisms, sparse information is available regarding starvation survival of oral microorganisms. An early report evaluated the survival of F. nucleatum in nutrient-limited media (Stuart medium) and showed that a low concentration of F. nucleatum cells (104 cfu/mL) was not recoverable after 1–2 days.24 In another study, albeit with a short observation period of 32 h, F. nucleatum was shown to have a limited capacity for survival in a nutrient-restricted medium, even at higher concentrations (109 cfu/mL), although starvation survival was dependent on prior growth rate.25 As for the other species, low concentrations of a eubacterium and peptostreptococcus species were reported to survive <1 day in Stuart medium,24 although at higher cell density (>107 cfu/mL) the species survived 8 and 13 days, respectively.24 The findings in these earlier reports are consistent with the present results showing an inability of these species to endure starvation or nutrient limitation.
The species F. nucleatum, P. anaerobius, P. alactolyticus and P. intermedia were selected for study because they are representative and often dominate the polymicrobial flora in untreated teeth, and yet they are rarely isolated by culture from root-filled teeth with persistent periapical lesions.11,12,18,21 In contrast, E. faecalis and C. albicans are opportunistic species frequently isolated from the canals of root-filled teeth with persistent lesions, but rarely in untreated root canal infections.26,27 The starvation-survival behavior of the former group varies significantly from the latter. Pooling the data from this and earlier studies16 (Richards et al, manuscript in preparation) illustrates the remarkable capacity of E. faecalis and C. albicans for growth in nutrient-limited conditions (1% and 5% serum) from a low initial density (103 cfu/mL) compared with the 4 species tested in this study, where even high cell numbers could not survive 21 days in 1% serum (Fig. 2). In serum-rich fluid (50%), the data on E. faecalis16 compared with P. alactolyticus (present study) highlights the marked difference in the minimum cell density required for growth (Fig. 3). In 50% PHS, just a few E. faecalis cells (101 cfu/mL) are enough for growth up to 106 cfu/mL, whereas P. alactolyticus, representative of the 4 species, requires at least 106 cfu/mL and shows a decline over time (Fig. 3).
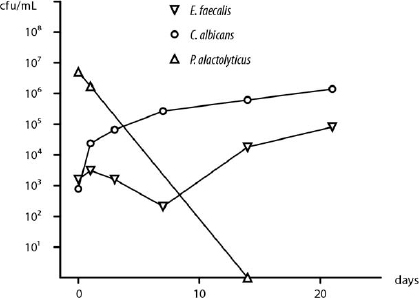
Fig. 2. Survival in nutrient-limited conditions. A representative species, P. alactolyticus (triangles, 1% serum) at high initial cell density (>106 cfu/mL) did not survive, compared with growth of Enterococcus faecalis (upside-down triangles, 1% serum)16 and Candida albicans (circles, 5% serum) (Richards et al., manuscript in preparation) from low cell numbers (103 cfu/mL).
Higher cell numbers at starvation onset positively influenced survival, which has also been reported for other species.16,28 One factor that plays a likely role is cell signaling, where cells collectively communicate that a critical density is reached (quorum sensing), so that as a community they maximize their chances for survival and pathogenesis.29,30 Nutrient limitation and high cell density are key characteristics of biofilm physiology,31 which raises 2 questions: What is the growth form in post-treatment infection, and does it influence starvation survival?
Ultrastructural investigations of the post-treatment microbial flora have shown growth in sessile biofilms, aggregates and planktonic forms.8-10 Immediately after treatment, some microorganisms may escape by harboring in isthmuses and branches of the root canal anatomy,10 although access to nutrition is likely to be limited or absent and many microbial communities in these ramifications probably face extinction. The in vitro results for F. nucleatum, P. anaerobius, P. alactolyticus and P. intermedia suggest that for these species this is their likely fate, although with a favorable location, other microbes with suitable properties may have the fortune to survive over time. In post-treatment disease, microbes have been observed as small biofilms, aggregates, planktonic forms, and occasionally in accessory canals as a larger community or as a limited invasion of dentinal tubules.8,9,32 Because all growth forms have been observed after treatment, we chose to study starvation survival of planktonic cells, which has advantages of simplicity, accuracy, and reproducibility.
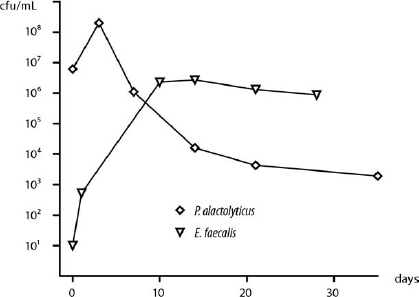
Fig. 3. Survival of species in serum-rich fluid. Cell numbers of a representative species, P. alactolyticus (diamonds, 50% serum), declined over time despite a high initial cell density (>106 cfu/mL), compared with growth of E. faecalis (upsidedown triangles, 50% serum)16 from a low initial number (101 cfu/mL).
Although biofilms are commonly regarded to be more resistant to killing by antimicrobial agents,31 there is evidence showing no difference between planktonic and biofilm forms and that it is in stationary phase where cells display maximum tolerance.33 In the present study, mature cultures essentially in stationary phase were harvested and starved, so it is reasonable to think that the same would be the case for starvation survival. Notwithstanding that analysis of starvation survival in the biofilm form is warranted, evidence from another oral species, Streptococcus mutans, has shown that starvation survival was more effective in batch cultures than in biofilms.34 The survival of a small population is probably related to the presence of persister cells33,35 in a low metabolic state.16
In ultrastructural studies of the post-treatment microbial flora,8-10 all cases show the presence of microorganisms in apical locations where they appear to have nutritional access, probably perfusion of a serum-like fluid, via the periapical tissues. How well do the 4 anaerobes use serum for survival? We found that the 4 tested species could not grow in serum unless the concentration was >10% and there was an initial cell density >106 cells/mL. These findings are consistent with earlier observations describing poor growth of F. nucleatum, P. anaerobius and P. intermedia in serum.5,6 Thus, available evidence suggests these anaerobes strongly depend on a nutrient-rich substrate and high cell numbers, preferably in a polymicrobial consortium, for growth. These conditions are more likely met in the untreated root canal infection, but would rarely be found in the root canal after treatment.
A number of studies have shown that after canal instrumentation, 25%-55% of cases still contain recoverable bacteria,36-41 although the remaining species are generally present in low cell numbers.38-41 Because the instrumented canal has essentially been stripped of a nutrient supply, those species that possess an adaptive capacity to endure long periods of nutrient limitation and to use limited serum-like fluid that may seep into the apical root canal will be best positioned to participate in post-treatment disease.
Cell survival was assessed by colony growth on plates. Although it could be argued that after starvation some cells may have so low a metabolic activity that they are unable to grow, i.e., they are viable but not culturable, this idea lacks incontrovertible proof,42-44 and it can be said that growth by culture remains the gold standard for assessment of cell viability. Alternative approaches such as cell staining hold the promise of highlighting viable cells by visible fluorescence of intact cell membranes, but they have their own limitations, such as nonspecific binding,45 and do not necessarily correlate with viability as shown by positive staining of formalin-fixed cells.34
The present study has shown that strict anaerobes of the type that frequently dominate the infection of untreated root canals do not have the capacity for survival in nutrient-limited or starvation conditions. In serum-rich fluid, and in high cell numbers, these species may survive; however, it is unlikely that these conditions exist in well treated root filled canals, which explains why these species are rarely isolated in persistent infections unless the canals are poorly treated.11,18 These findings also explain why bacteria left in the root canal will in some cases die and in other cases regrow.46 In contrast, E. faecalis and C. albicans, which are characteristic of species found in persistent post-treatment infection, are well equipped to survive in the root-filled canal because they have the capacity to survive long periods of starvation or nutrient limitation and can survive or recover from low numbers (Richards et al, manuscript in preparation).16
In summary, nutrient limitation appears to be a compelling selection factor in the root-filled canal. We have shown that species that typically dominate the polymicrobial infection, F. nucleatum, P. anaerobius, P. alactolyticus and P. intermedia, lack the capacity for starvation survival, in contrast to E. faecalis and C. albicans, which are well adapted for starvation survival. Thus, species isolated from cases with post-treatment disease appear to possess a capacity for starvation survival, unlike the strict anaerobes that dominate infection in untreated cases, which helps explain why the latter group rarely participate in post-treatment disease.
The authors thank Mrs. Chrissie Roth for excellent technical assistance.
REFERENCES
1. Loesche WJ. Importance of nutrition in gingival crevice microbial ecology. Periodontics 1968;6:245-9.
2. Grenier D, Mayrand D. Nutritional relationships between oral bacteria. Infect Immun 1986;53:616-20.
3. Carlsson J. Microbiology of plaque associated periodontal disease. In: Lindhe J, editor. Textbook of clinical periodontology. Copenhagen: Munksgaard: 1990. p. 129-52.
4. Fabricius L, Dahlén G, Holm SE, Möller ÅJR. Influence of combinations of oral bacteria on periapical tissues of monkeys. Scand J Dent Res 1982;90:200-6.
5. ter Steeg PF, Van der Hoeven JS, de Jong MH, van Munster PJ, Jansen MJ. Enrichment of subgingival microflora on human serum leading to accumulation of Bacteroides species, Peptostreptococci and Fusobacteria. Antonie Leeuwenhoek 1987;53:261-72.
6. ter Steeg PF, van der Hoeven JS. Development of periodontal microflora on human serum. Microb Ecol Health Dis 1989;2:1-10.
7. Sundqvist G. Associations between microbial species in dental root canal infections. Oral Microbiol Immunol 1992;7:257-62.
8. Nair PNR, Sjögren U, Krey G, Kahnberg K-E, Sundqvist G. Intraradicular bacteria and fungi in root-filled, asymptomatic human teeth with therapy-resistant periapical lesions: a long-term light and electron microscopic follow-up study. J Endod 1990;16:580-8.
9. Nair PNR, Sjögren U, Figdor D, Sundqvist G. Persistent periapical radiolucencies of root-filled human teeth, failed endodontic treatments, and periapical scars. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999;87:617-27.
10. Nair PNR, Henry S, Cano V, Vera J. Microbial status of apical root canal system of human mandibular first molars with primary apical periodontitis after “one-visit” endodontic treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005;99:231-52.
11. Sundqvist G, Figdor D, Persson S, Sjögren U. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1998;85:86-93.
12. Molander A, Reit C, Dahlén G, Kvist T. Microbiological status of root-filled teeth with apical periodontitis. Int Endod J 1998;31:1-7.
13. Peciuliene V, Balciuniene I, Eriksen HM, Haapasalo M. Isolation of Enterococcus faecalis in previously root-filled canals in a Lithuanian population. J Endod 2000;26:593-5.
14. Hancock HH, III, Sigurdsson A, Trope M, Moiseiwitsch J. Bacteria isolated after unsuccessful endodontic treatment in a North American population. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2001;91:579-86.
15. Peciuliene V, Reynaud AH, Balciuniene I, Haapasalo M. Isolation of yeasts and enteric bacteria in root-filled teeth with chronic apical periodontitis. Int Endod J 2001;34:429-34.
16. Figdor D, Davies JK, Sundqvist G. Starvation survival, growth and recovery of Enterococcus faecalis in human serum. Oral Microbiol Immunol 2003;18:234-9.
17. Cheung GS, Ho MW. Microbial flora of root canal–treated teeth associated with asymptomatic periapical radiolucent lesions. Oral Microbiol Immunol 2001;16:332-7.
18. Pinheiro ET, Gomes BP, Ferraz CC, Sousa EL, Teixeira FB, Souza-Filho FJ. Microorganisms from canals of root-filled teeth with periapical lesions. Int Endod J 2003;36:1-11.
19. Sundqvist G. Bacteriological studies of necrotic dental pulps. In: Umeå University Odontological Dissertations No. 7. Umeå, Sweden: Umeå University; 1976.
20. Siqueira JF Jr., Rôças IN, Paiva SS, Magalhães KM, Guimarães-Pinto T. Cultivable bacteria in infected root canals as identified by 16S rRNA gene sequencing. Oral Microbiol Immunol 2007;22:266-71.
21. Pinheiro ET, Gomes BP, Ferraz CC, Teixeira FB, Zaia AA, Souza Filho FJ. Evaluation of root canal microorganisms isolated from teeth with endodontic failure and their antimicrobial susceptibility. Oral Microbiol Immunol 2003;18:100-3.
22. Siqueira JF Jr., Rôças IN. Polymerase chain reaction-based analysis of microorganisms associated with failed endodontic treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2004;97:85-94.
23. Sakamoto M, Siqueira JF Jr., Rôças IN, Benno Y. Molecular analysis of the root canal microbiota associated with endodontic treatment failures. Oral Microbiol Immunol 2008;23:275-81.
24. Möller ÅJR. Microbiological examination of root canals and periapical tissues of human teeth. Methodological studies. Odontol Tidsk 1966;74(Suppl):1-380.
25. Rogers AH, Zilm PS. The influence of intracellular polyglucose and prior growth rate on the survival of Fusobacterium nucleatum under starvation conditions. Oral Microbiol Immunol 1995;10:119-21.
26. Siqueira JF Jr., Rôças IN, Souto R, de Uzeda M, Colombo AP. Actinomyces species, streptococci, and Enterococcus faecalis in primary root canal infections. J Endod 2002;28:168-72.
27. Sundqvist G, Figdor D. Life as an endodontic pathogen. Ecological differences between the untreated and root-filled root canals. Endod Topics 2003;6:3-28.
28. Watson SP, Clements MO, Foster SJ. Characterization of the starvation-survival response of Staphylococcus aureus. J Bacteriol 1998;180:1750-8.
29. Lazazzera BA. Quorum sensing and starvation: signals for entry into stationary phase. Curr Opin Microbiol 2000;3:177-82.
30. de Kievit TR, Iglewski BH. Bacterial quorum sensing in pathogenic relationships. Infect Immun 2000;68:4839-49.
31. Fux CA, Costerton JW, Stewart PS, Stoodley P. Survival strategies of infectious biofilms. Trends Microbiol 2005;13:34-40.
32. Ricucci D, Siqueira JF Jr. Anatomic and microbiologic challenges to achieving success with endodontic treatment: a case report. J Endod 2008;34:1249-54.
33. Spoering AL, Lewis K. Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobials. J Bacteriol 2001;183:6746-51.
34. Renye JA Jr., Piggot PJ, Daneo-Moore L, Buttaro BA. Persistence of Streptococcus mutans in stationary-phase batch cultures and biofilms. Appl Environ Microbiol 2004;70:6181-7.
35. Keren I, Kaldalu N, Spoering A, Wang Y, Lewis K. Persister cells and tolerance to antimicrobials. FEMS Microbiol Lett 2004;230:13-8.
36. Byström A, Sundqvist G. Bacteriologic evaluation of the effect of 0.5 percent sodium hypochlorite in endodontic therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1983;55:307-12.
37. Byström A, Sundqvist G. The antibacterial action of sodium hypochlorite and EDTA in 60 cases of endodontic therapy. Int Endod J 1985;18:35-40.
38. Sjögren U, Sundqvist G. Bacteriologic evaluation of ultrasonic root canal instrumentation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1987;63:366-70.
39. McGurkin-Smith R, Trope M, Caplan D, Sigurdsson A. Reduction of intracanal bacteria using GT rotary instrumentation, 5.25% NaOCl, EDTA, and Ca(OH)2. J Endod 2005;31:359-63.
40. Vianna ME, Horz HP, Gomes BP, Conrads G. In vivo evaluation of microbial reduction after chemo-mechanical preparation of human root canals containing necrotic pulp tissue. Int Endod J 2006;39:484-92.
41. Siqueira JF Jr., Rôças IN, Paiva SS, Guimarães-Pinto T, Magalhães KM, Lima KC. Bacteriologic investigation of the effects of sodium hypochlorite and chlorhexidine during the endodontic treatment of teeth with apical periodontitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007;104:122-30.
42. Bogosian G, Morris PJ, O’Neil JP. A mixed culture recovery method indicates that enteric bacteria do not enter the viable but nonculturable state. Appl Environ Microbiol 1998;64:1736-42.
43. Bogosian G, Aardema ND, Bourneuf EV, Morris PJ, O’Neil JP. Recovery of hydrogen peroxide–sensitive culturable cells of Vibrio vulnificus gives the appearance of resuscitation from a viable but nonculturable state. J Bacteriol 2000;182:5070-5.
44. Bogosian G, Bourneuf EV. A matter of bacterial life and death. EMBO Rep 2001;2:770-4.
45. Biggerstaff JP, Le Puil M, Weidow BL, Prater J, Glass K, Radosevich M, et al. New methodology for viability testing in environmental samples. Mol Cell Probes 2006;20:141-6.
46. Byström A, Sundqvist G. Bacteriologic evaluation of the efficacy of mechanical root canal instrumentation in endodontic therapy. Scand J Dent Res 1981;89:321-8.
Reprint requests:
Malin Brundin, DDS
Department of Odontology/Endodontics
Faculty of Medicine
Umeå University
Umeå, 90187
Sweden
aDepartment of Odontology/Endodontics, Faculty of Medicine, Umeå University.
bDepartment of Microbiology, Monash University.
Received for publication Mar 2, 2009; accepted for publication Mar 6, 2009.
1079-2104/$ - see front matter
© 2009 Published by Mosby, Inc.
doi:10.1016/j.tripleo.2009.03.018